
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
| For the fiscal year ended August 31, 2019 |
|
|
or | |
|
|
¨ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
| For the transition period from [ ] to [ ] |
Commission file number 000-52138
LEXARIA BIOSCIENCE CORP. |
(Exact name of registrant as specified in its charter) |
Nevada |
| 20-2000871 |
State or other jurisdiction of incorporation or organization |
| (I.R.S. Employer Identification No.) |
|
|
|
#100 – 740 McCurdy Road, Kelowna BC Canada |
| V1X 2P7 |
(Address of principal executive offices) |
| (Zip Code) |
Registrant’s Telephone number, including area code: 250-765-6424
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
| Name of Each Exchange On Which Registered |
N/A |
| N/A |
Securities registered pursuant to Section 12(b) of the Act:
Title of Class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock, Par Value $0.001 | LXRP LXX | OTCQX CSE |
Indicate by check mark if the registered is a well-known seasonal issuer, as defined in Rule 405 the Securities Act Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the last 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically, every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-K (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ¨ | Accelerated filer | x |
Non-accelerated filer | ¨ | Smaller reporting company | x |
| Emerging growth company | ¨ |
If an emerging growth company, indicate by a check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of Common Stock held by non-affiliates of the Registrant on February 28, 2019 was $83,889,033 based on the average of the high and low bid and asked price of the Registrant’s shares of common stock on the OTCQX or $1.42 on February 28, 2019.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock as of the latest practicable date.
78,787,134 common shares as of November 5, 2019
DOCUMENTS INCORPORATED BY REFERENCE
None.

| Page 2 of 83 |
PART 1
Cautionary Note Regarding Forward-Looking Statements
This annual report contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. Any statements contained herein that are not statements of historical fact may be forward-looking statements. These statements relate to future events or our future financial performance. Any forward-looking statements are based on our present beliefs and assumptions as well as the information currently available to us. In some cases, you can identify forward-looking statements by terminology such as “may”, “will”, “should”, “could”, “targets”, “goal”, “expects”, “plans”, “anticipates”, “believes”, “estimates”, “predicts”, “potential” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks in the section entitled “Risk Factors” set forth in Item 1(A) in this report on Form 10-K, that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. We caution you not to place undue reliance on any forward-looking statements as they speak only as of the date on which such statements were made, and we undertake no obligation to update any forward-looking statement or to reflect the occurrence of an unanticipated event. New factors may emerge and it is not possible to predict all factors that may affect our business and prospects. Further, management cannot assess the impact of each factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
Our audited annual consolidated financial statements are stated in United States Dollars and are prepared in accordance with United States Generally Accepted Accounting Principles (US GAAP). The following discussion should be read in conjunction with our financial statements and the related notes that appear elsewhere in this report.
In this annual report, unless otherwise specified, all dollar amounts are expressed in United States dollars. All references to “C$” or “CDN$” refer to Canadian dollars and all references to “common shares” and “shares” refer to the common shares in our capital stock, unless otherwise indicated.
As used in this quarterly report, the terms “Lexaria” “we”, “us”, “our” and “Company” mean Company and/or our subsidiaries, unless otherwise indicated.
General and Historical Overview of Our Business
The Company was formed on December 9, 2004 under the laws of the State of Nevada as an independent oil and gas company engaged in the exploration, development and acquisition of oil and gas properties in the United States and Canada. In March of 2014, the Company began its entry into the bioscience and alternative health and wellness business and discontinued its involvement in the oil and gas business in November 2014. In May 2016, the Company also commenced out-licensing its patented DehydraTECH™ technology (the “Technology”) for improved delivery of bioactive compounds that promotes healthy ingestion methods, lower overall dosing and higher effectiveness in active molecule delivery. The Company has its office in Kelowna, BC, Canada.
Our common stock is quoted on the OTCQX under the symbol “LXRP” and on the Canadian Securities Exchange under the symbol “LXX”.

| Page 3 of 83 |
| Table of Contents |
In 2014, the Company changed its business direction to enter into the hemp oil-based food supplement industry in the US and the legal medical marijuana industry in Canada via a 49% interest in a joint venture arrangement. The 49% interest was subsequently sold on June 26, 2015 for $4,900 by the Company to focus on its food sciences activities.
The Company’s food sciences activities include the development of our proprietary nutrient infusion technologies for the production of functional foods, and the production of enhanced food products under our consumer product brands, ViPova™, Lexaria Energy™, TurboCBD™ and ChrgD+TM. The Company’s patented lipid nutrient infusion DehydraTECH technology is believed to improve taste, rapidity and delivery of bioactive compounds that include cannabinoids, vitamins, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), nicotine and other molecules compared to what is possible without lipophilic enhancement technology. This can allow for lower overall dosing requirements and/or higher effectiveness in active molecule delivery.
We maintain our registered agent’s office and our U.S. business office at Nevada Agency and Transfer Company, 50 West Liberty, Suite 880, Reno, Nevada 89501. Our telephone number is (755) 322-0626.
The address of our principal executive office is Unit 100–740 McCurdy Road, Kelowna BC V1X 2P7. We have administrative functions located in Phoenix, Arizona. Our main corporate website is located at www.lexariabioscience.com.
Due to the implementation of British Columbia Instrument 51-509 on September 30, 2008, by the British Columbia Securities Commission, we have been deemed to be a British Columbia based reporting issuer. As such, we are required to file certain information and documents at www.sedar.com.
Available Information
We file annual, quarterly and current reports, proxy statements and other information with the Commission. These filings are available to the public on the Internet at the Commission's website at http://www.sec.gov.
Our Internet address is http://www.lexariabioscience.com/ (this website address is not intended to function as a hyperlink and the information contained on our website is not intended to be a part of this Report).
We make available free of charge on http://www.lexariabioscience.com/investors/regulatory-filings/ our annual, quarterly and current reports, and amendments to those reports, as soon as reasonably practical after we electronically file such material with, or furnish it to, the Commission. We may from time to time provide important disclosures to investors by posting them in the Investor Relations section of our website, as allowed by the Commission's rules. The information on the website listed above is not and should not be considered part of this Report and is intended to be an inactive textual reference only.
Our Current Business
Our business plan is currently focused on the development of strategic partnerships with licensees for our patented Technology in exchange for up front and/or staged licensing fees over time. Secondarily and more generally, we continue to investigate national and international opportunities for development and distribution of the Company’s enhanced functional food and supplement product offerings; to investigate expansions and additions to our intellectual property portfolio; and, to search for additional opportunities in alternative health sectors. This includes the acquisition or development of intellectual property if and when we believe it is advisable to do so.
Our current patent portfolio includes patent family grants relating to: Infused Food and Beverage Compositions and Methods of Use Thereof, pertaining to Lexaria’s method of improving bioavailability and taste, and the use of DehydraTECH technology as a delivery platform for a wide variety of Active Pharmaceutical Ingredients (“APIs”) encompassing all cannabinoids including tetrahydrocannabinol (“THC”); fat soluble vitamins; Non-Steroidal Anti-Inflammatory Drugs (“NSAIDs”) pain medications; and nicotine.
Lexaria hopes to reduce other common but less healthy administration methods, such as smoking, as it embraces the benefits of its technology for public health. The Company is aggressively pursuing patent protection in national jurisdictions around the world. The Company currently has more than 55 patent applications pending worldwide and, due to the complexity of pursuing patent protection, the quantity of patent applications will vary continuously as each application advances or stalls. Lexaria is also filing new patent applications for novel new discoveries that arise from the Company’s R&D programs and, due to the inherent unpredictability of scientific discovery, it is not possible to predict if or how often such new applications might be filed.

| Page 4 of 83 |
| Table of Contents |
During the past fiscal year the Company experienced the following significant corporate developments:
On September 7, 2018, the Company announced additions to its patent portfolio with three new Australian patents granted to Lexaria by the Australian Patent Office. The three Australian patents are projected to expire on June 10, 2035.
The USPTO also issued two new Notices of Allowance for pending patent applications and the Company announced the grants on October 16, 2018. The two new patents are related to certain cannabinoid infused beverage compositions utilizing Lexaria’s proprietary DehydraTECH process. Newly granted patent numbers US 10,103,225 B2 and US 10,084,044 B2 provide protection for compositions as well as methods for making the compositions, each of which include the use of both non-psychoactive cannabinoids such as cannabidiol (“CBD”) and also psychoactive cannabinoids such as THC. The Company holds fourteen issued patents within its first patent family, “Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof”, one in the second patent family “Methods for Formulating Orally Ingestible Compositions Comprising Lipophilic Active Agents”, and one in the third patent family “Stable Ready-to-Drink Beverage Compositions Comprising Lipophilic Active Agents” that significantly strengthen Lexaria’s intellectual property claims in the US and Australia. Of note, issuance of these patents in the second and third patent families represents the first time the Company has been granted claims for use of its technology in connection with the treatment of specific diseases and medical conditions affecting humans, which the Company believes will prove to be of significance to the pharmaceutical industry sector as it further develops and grows. We continue to pursue claims in corresponding pending applications around the world.
On September 7, 2018, the Company announced the filing of a new strategic patent application. The new provisional patent application is entitled “Lipophilic Active Agent Infused Tobacco Leaves and/or Tobacco Materials and Methods of Use Thereof”. This application represents Lexaria’s tenth patent family and expands the applicability of the already-patented DehydraTECH process to impart benefits to tobacco leaves that may be utilized to deliver compounds that may or may not include nicotine.
On October 10, 2018, the Company announced completed the creation of four wholly-owned subsidiary companies. This new corporate structure more suitably reflects the distinct customer bases and business applications for each subsidiary, thereby allowing the Company to focus its future research and consider financing structures and industry partnerships specifically optimized to each.
| · | Lexaria CanPharm ULC, a Canadian company focused on providing DehydraTECH technology and other enhancements to the global cannabis industry. |
|
|
|
| · | Lexaria Nicotine LLC, a US company with a global license to provide DehydraTECH technology to the global nicotine and tobacco industries. |
|
|
|
| · | Lexaria Hemp Corp., a US company globally licensed to provide DehydraTECH to the rapidly growing hemp-based foods and supplements industries. |
|
|
|
| · | Lexaria Pharmaceutical Corp., a US company globally empowered to license DehydraTECH to the large and diverse pharmaceutical sectors. |
On November 13, 2018, the Company announced the launch of ChrgD+, a water-soluble, ready-mix hemp supplement powder packet formulation designed to be added to any drink. Lexaria engaged Cultivating Wellness Inc., a California-based brand development and distribution company, to create the ChrgD+ premium brand. Cultivating Wellness’ distribution network reaches tens of thousands of retail buyers in c-stores, grocery chains, specialty retail, and national accounts. We subsequently launched our retail e-commerce website for public consumers in June of 2019.
On November 26, 2018, the Company announced it submitted a research application under Health Canada’s Cannabis Tracking and Licensing System for the operation of a Kelowna-based R&D laboratory within Lexaria’s new head office. The laboratory’s creation enhances Lexaria’s ability to formulate for analytical purposes, various products that may contain cannabinoids or other controlled substances. Experimental work on nicotine formulations, nonsteroidal anti-inflammatory drugs, vitamins and other bioactive compounds of interest began soon after completion of lab construction with work on cannabinoid related formulations occurring after receipt of Lexaria’s research license. Bringing formulation work in-house enables the Company to expand its work schedules while reducing costs and development timelines.

| Page 5 of 83 |
| Table of Contents |
On January 15, 2019, the Company announced that its wholly-owned subsidiary Lexaria Nicotine LLC (“Lexaria Nicotine”) and Altria Ventures Inc., an indirect wholly-owned subsidiary of Altria Group, Inc. (“Altria”), executed definitive agreements to pursue innovation in oral, reduced risk nicotine consumer products using Lexaria’s patented DehydraTECH technology. Altria is funding a milestone-based research & development program (“R&D Program”) in exchange for a minority equity interest in Lexaria Nicotine and certain DehydraTECH license rights. Altria will provide initial funding of $1 million, with the option for additional funding of up to $12 million total through multiple phased private financings. Altria was granted a license to use Lexaria Bioscience’s DehydraTECH technology for oral nicotine delivery forms on an exclusive basis in the United States and a non-exclusive basis elsewhere globally. Altria will pay Lexaria Nicotine a royalty on revenue generated from the sale of all nicotine products containing DehydraTECH, until such time it may acquire 100% ownership in Lexaria Nicotine. Altria will initially have the right to appoint one of the seven managers of Lexaria Nicotine and, through the additional phased investments, may have the right to appoint up to three of the seven managers. Altria has the option to acquire 100% ownership interest in Lexaria Nicotine commensurate with then-current fair market value. Altria gained no rights of ownership to Lexaria Bioscience and has no rights of board of director representation on Lexaria Bioscience
On February 21, 2019, the Company announced additional findings upon completion of further data analyses from its 2018 randomized, placebo-controlled, double-blinded European human clinical study that evaluated TurboCBD, the Company’s proprietary, DehydraTECH powered, CBD fortified hemp-oil capsule. A single 90mg dose of TurboCBD provided evidence of lower blood pressure; higher blood flow to the brain; faster delivery onset of CBD into the bloodstream; and, larger quantities of CBD within the blood compared to a single 90mg dose of generic CBD.
Key metabolic and hemodynamic performance findings linked to bioavailability enhancements were revealed in the study, which compared a 90 mg dose of Lexaria’s TurboCBD to a 90 mg dose without Lexaria’s DehydraTECH technology (the “positive control”) as well as a placebo, as follows:
· Analysis of mean arterial blood pressure (MAP) at peak blood levels of CBD achieved with Lexaria’s TurboCBD demonstrated a significant reduction in MAP compared to placebo (95% CI; p=0.027). This finding was not observed with the dose-matched positive control formulation for which there was no significant decrease in MAP compared to placebo (95% CI; p=0.625); · Cerebral perfusion was also analysed by an index of conductance in the middle cerebral artery (MCA). The findings revealed that Lexaria’s TurboCBD caused the greatest increase in MCA conductance relative to both the positive control formulation and placebo (95% CI; p=0.017 and P=0.002 respectively);
Finally, over the six-hour study, analysis of the total area under the curve (AUC) demonstrated that Lexaria’s TurboCBD resulted in a notable trend for higher levels of CBD in the bloodstream overall than the positive control formulation with total AUC of 10,865 ± 6,322 observed with Lexaria’s formulation compared to 7,115 ± 2,978 observed with the positive control (95% CI; p=0.096). Furthermore, when normalized to body mass, the AUC at the peak CBD concentration was markedly and significantly (95% CI; p=0.02) higher with the TurboCBD 90 mg dose compared to the 90 mg dose positive control formulation.
On March 20, 2019, the Company announced an in vivo research program to test Lexaria designed nanotech enhancements comprised of eleven separate animal studies. Lexaria also announced that, effective March 15, 2019, it terminated the definitive license agreement entered into between Lexaria CanPharm ULC and NeutriSci International Inc. that was originally announced on February 26, 2018.
On April 24, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Canpharm ULC, to provide Lexaria’s patented DehydraTECH technology to a private California-based company for its utilization in certain CBD-based beverages to be produced and sold in California and Nevada that may include any combination of ready-to-drink beverages such as non-alcoholic beers, wines and spirits; cold or hot coffee or teas, sports drinks and more.
On May 7, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Hemp Corp., to provide Lexaria’s patented DehydraTECH technology to a private Nevada-based company for its utilization in certain CBD-based beverages to be produced and sold across the USA that may include any combination of ready-to-drink beverages such as non-alcoholic beers, wines and spirits; cold or hot coffee or teas, sports drinks and more.

| Page 6 of 83 |
| Table of Contents |
On May 15, 2019, the Company released initial results from its research program announced March 2019 demonstrating measurable quantities of cannabidiol into blood in as little as 2 minutes. In each arm of the Lexaria animal studies, 10 male Sprague-Dawley rats were administered CBD at 25mg per kg of bodyweight. Delivery of CBD into the bloodstream was monitored over a 60-minute duration. In the first animal study results, Lexaria compared its standard DehydraTECH formulation that combines cannabinoids with long-chain fatty acids (“LCFA”) using Lexaria’s patented dehydration processing technique to a concentration-matched formulation utilizing coconut oil which is a commonly used medium chain triglyceride (“MCT”) oil in the cannabis edibles industry.
· At 2 minutes DehydraTECH’s LCFA formulation delivered measurable CBD in blood, compared to no measurable CBD in blood until 6 minutes and onwards for the MCT oil formulation. · At 15 minutes DehydraTECH’s LCFA formulation achieved a CBD blood concentration level that was 475% more than the MCT oil formulation; and, the DehydraTECH LCFA formulation CBD blood levels reached at 15 minutes were greater than the CBD blood levels reached by the MCT oil formulation at any time point during the 60-minute evaluation. · At 60 minutes DehydraTECH’s LCFA formulation achieved a CBD blood concentration level of 319% more than the MCT oil formulation. · Over the entire 60-minute study, the animals that received the standard DehydraTECH LCFA formulation achieved an average maximum CBD blood concentration level that was 334% more than the average maximum blood concentration level of the animals that received the MCT oil formulation (p<0.0021). · Over the entire 60-minute study, the area under the curve (AUC) (total quantity of CBD delivered) for the Lexaria DehydraTECH LCFA formulation was 389% more than the MCT oil formulation (p<0.0011).
Lexaria also tested for brain tissue concentrations to quantify 8-hour CBD delivery from the DehydraTECH-enabled LCFA formulation compared to the MCT oil formulation and DehydraTECH’s LCFA formulation outperformed the MCT oil formulation by 246%.
On May 21, 2019, the Company announced a major expansion in operations by Nuka Enterprises LLC, (“Nuka”) maker of “1906” brand edibles over the next two years into Illinois, Ohio, Massachusetts, Michigan and other states. The comprehensive semi-exclusive agreement provides Nuka and 1906 with competitive technological advantages until 2028. A second license provides Nuka and 1906 with the immediate ability to utilize DehydraTECH technology for CBD across the US marketplace.
On May 28, 2019, the Company released additional results from its research program wherein animal testing proved that combining Lexaria’s DehydraTECH delivery technology with generic nanotech techniques delivers 1,137% more cannabidiol into animal brain tissue following oral ingestion than certain existing industry formulations. Lexaria combined its DehydraTECH delivery technology with a standard form of nanotechnology and analyzed subsequent delivery into brain tissue following oral ingestion. In each arm of the Lexaria animal studies, 10 male Sprague-Dawley rats were orally administered CBD at the rate of 25mg per kg of bodyweight. Delivery of CBD into the brain was reported 8 hours after dosing.
| · | The Lexaria DehydraTECH LCFA formulation without nanotech achieved an average brain tissue accumulation level that was 246% higher than the average for those animals that received the MCT oil formulation (p=0.0013). |
|
|
|
| · | The Lexaria DehydraTECH LCFA formulation with nanotech achieved an average brain tissue accumulation level that was 1,137% higher than the average for those animals that received the MCT oil formulation (p=0.0178). |
On June 4, 2019 the Company announced additional results from the March 20, 2019 announced animal studies demonstrating improved performance characteristics resulting in new patent applications. This arm of the study tested DehydraTECH delivery technology with compounds postulated to behave in a synergistic fashion for enhancement of gastro-intestinal absorption separate and distinct from nanotech techniques.
On July 10, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Hemp Corp, to provide Lexaria’s patented DehydraTECH technology to Nic’s Beverages Ltd for use in CBD-based beverages to be produced and sold throughout the United States.
On July 11, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Hemp Corp., to provide Lexaria’s patented DehydraTECH technology to Universal Hemp LLC, a B2B manufacturing company of hemp-derived bulk ingredients to the nutraceutical and consumer packaged goods industries to be produced and sold across the USA immediately, and in Canada when regulations permit. Agreed to minimum payments over the life of the 5-year agreement are $3,750,000.

| Page 7 of 83 |
| Table of Contents |
On July 24, 2019, the Company announced that it entered a 10-year Joint Manufacturing Partnership (JMP) with Hill Street Beverages Company Inc. to produce commercial products including processed THC cannabis and/or CBD hemp powder including among other categories; tablets, capsules, or packets for sale in Canada and for export where permitted. The JMP will also produce similar powders as a bulk ingredient for manufacturing processes for sale to other licensed producers seeking to use DehydraTECH to create their own products for sale within Canada. Profits from this business unit will be shared equally between Hill Street and Lexaria. In addition to the JMP, Hill Street acquired two global semi-exclusive licenses (with minor exceptions) to utilize Lexaria’s DehydraTECH THC beverage infusion technology around the world, valid for 10 years. Under the terms of the agreements, Hill Street will pay an annual licensing fee and up to an additional $1,800,000 to Lexaria by issuing $800,000 in common shares of Hill Street to Lexaria initially, and Lexaria will issue $250,000 in restricted common shares to Hill Street. In addition, Hill Street will issue up to an additional $500,000 in shares of Hill Street when they enter each of the first two international markets subject to TSXV and CSE approval, as applicable. Pursuant to the terms of the JMP agreements, Lexaria will issue an aggregate $250,000 in restricted common shares to Hill Street. Closing of the Hill Street / Lexaria agreements is subject to normal regulatory approvals and the closing of the Hill Street / OneLeaf transaction announced by Hill Street. Subsequent to August 31, 2019, Hill Street has been unable to close its transaction with OneLeaf and is currently searching for an alternate location from which to base the Hill Street / Lexaria agreements, thus there is a possibility that this transaction may not close as expected.
On August 8, 2019, the Company announced the successful completion of its Master Collaborative Research Agreement (“the R&D Program”) with the National Research Council of Canada to investigate technical aspects and new opportunities associated with bioavailability enhancement of lipophilic active ingredient compositions using Lexaria’s patented DehydraTECH technology. The R&D Program determined that Lexaria’s DehydraTECH does not create a covalent-bonded new molecular entity. The R&D program also tested Lexaria’s formulations at highly acidic levels of pH 1.12, higher than many flavored beverages that have pH levels between 2.73 to 3.05, and mildly acidic levels of pH 4.82, and reports no chemical modification or presence of degradation of the active pharmaceutical ingredients for both of the formulation classes analysed in this aspect of the program: cannabinoids and nicotine polacrilex.
On August 8, 2019, the Company announced that its subsidiary, Lexaria CanPharm ULC., has been issued cannabis Research and Development (“R&D”) license LIC-7NONT76UNW-2019 by Health Canada with a four-year term until August 9, 2023 unless renewed.
On August 14, 2019, the Company announced four patent grants. Australia Patent #2016367036 Grant Date July 30, 2019 – Methods for formulating orally ingestible compositions comprising lipophilic active agents, Australia Patent #2018220067 Grant Date July 30, 2019 – Food and beverage compositions infused with lipophilic active agents and methods of use thereof, US Patent #10,374,036 Grant Date August 6, 2019 - Food and beverage compositions infused with lipophilic active agents and methods of use thereof, and US Patent #10,381,440 / Grant Date August 13, 2019 - Food and beverage compositions infused with lipophilic active agents and methods of use thereof.
On August 22, 2019, the Company announced the online commercial launch of ChrgD+, a water-soluble multi-spectrum hemp oil in a powdered format with our DehydraTECH technology.
On August 22, 2019, the Company announced a patent granted in Australia: #2016367037 Grant Date August 15, 2019 – “Stable ready-to-drink beverage compositions comprising lipophilic active agents”.
The Company experienced the following significant corporate developments subsequent to August 31, 2019
On September 17, 2019, the Company announced that final study results of the 2018 human clinical study evaluating CBD delivery and effectiveness using its patented DehydraTECH powered TurboCBD capsules have been published in the peer reviewed medical journal, “Advances in Therapy”. Advances in Therapy focuses on clinical medicine and pharmaceutical research and has been published continually since 1984.
The study was conducted and well tolerated in 12 healthy young male athletes and the investigators concluded that further studies are warranted.

| Page 8 of 83 |
| Table of Contents |
Food Science and Technology
Lexaria is a biotechnology and food science company focused on developing and out-licensing its proprietary technology for improved taste, rapidity, and delivery of bioactive compounds in foods and other ingestible products Lexaria is focusing its capital and management time on its pursuit of intellectual property, technology licensing opportunities, an expanding portfolio of patent pending applications, and functional food and supplement formulations.
On November 11, 2014, the Company acquired 51% of PoViva Tea LLC (100% October 23, 2017) and executed an operating agreement to develop a business of legally producing, manufacturing, importing/exporting, testing, researching and developing, a line of hemp oil with cannabidiol-infused teas, drinks and foods. Lexaria oversees all aspects of the business including, but not limited to, production, product quality, licensing, testing, product legality, accounting, marketing, capital investment, capital raising, sales, branding, advertising and fulfillment.
The Company introduced an expanding variety of hemp fortified consumer food products throughout 2015 to demonstrate Lexaria’s DehydraTECH technology to both consumers and potential licensees. From January 2015 to December 2015, seven (7) flavors of teas; hot chocolate; coffee, and two (2) flavors of protein energy bars were introduced – all utilizing Lexaria’s patented technology DehydraTECH for the more palatable and efficient delivery of bioactive molecules infused within those food products.
In the production of the products, for each raw material to be used in ViPova branded products, the Company assesses if the product inputs and the completed products comply with all applicable food and drug laws, and that the inputs and the finished products meet all applicable legal and quality standards including and as it relates to hemp oil content; THC content; molds and mildews; heavy metals; and may measure additional components.
The US Federal government, through the US Department of Health and Human Services, owns US Patent #6630507, which among other things, claims that
“Cannabinoids have been found to have antioxidant properties, unrelated to NMDA receptor antagonism. This new found property makes cannabinoids useful in the treatment and prophylaxis of wide variety of oxidation associated diseases, such as ischemic, age-related, inflammatory and autoimmune diseases. The cannabinoids are found to have particular application as neuroprotectants, for example in limiting neurological damage following ischemic insults, such as stroke and trauma, or in the treatment of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease and HIV dementia.”
For reference, cannabinoids are compounds that affect cannabinoid receptors located on many human cells. CB1 receptors are widely found within the human brain; and CB2 receptors are found with the human immune system and have been linked to anti-inflammatory and other responses.
Despite independent scientific findings in many locations around the world, some regulatory agencies do not officially recognize that a human endocannabinoid system exists.
Over one hundred different cannabinoids have been isolated from the cannabis plant, most of which do not have psychoactive properties. One that does have psychoactive properties is THC. Endocannabinoids are produced naturally in the human body while Phyto cannabinoids are produced in several plant species, most abundantly in the Cannabis plant.
CBD is one of the major Phyto cannabinoid forms of cannabinoids and is not psychoactive, often contributing more than 35% of the extracts from the cannabis plant resin. CBD occurs naturally in other plant species beyond cannabis. For example, the most widely acknowledged alternative source of Phyto cannabinoid is in the better understood Echinacea species, in widespread use as a dietary supplement. Most Phyto cannabinoids are virtually insoluble in water but are soluble in lipids and alcohol. The World Anti Doping Agency (“WADA”) has exempted CBD from its 2018 list of banned substances.
The Alternative Health sector is large and growing. A long-term Medical Expenditure Panel Survey was conducted from 2002 until 2008 with at least 29,370 subjects asked repeatedly if they had seen any kind of health care practitioner in the previous six months. The survey recorded whether the health care provider was a “complementary and alternative medicine care professional,” including “homeopathic, naturopathic, or herbalist.”

| Page 9 of 83 |
| Table of Contents |
Between 5.3% and 5.8% of the survey group at any one time reported that they had seen a complementary or alternative medicine provider. Based on the US population of ~328,000,000, this suggests between 17.4 million and 19.0 million Americans are seeking an alternative health care professional at any given time.
Meanwhile the Centers for Disease Control and Prevention, in an April 2011 NCHS Data Brief, reported that more than 50% of the population uses dietary supplements of one kind or another. Detailed findings from that report included:
· Use of dietary supplements is common among the U.S. adult population. Over 40% used supplements in 1988–1994, and over one-half in 2003–2006. · Multivitamins/multiminerals are the most commonly used dietary supplements, with approximately 40% of men and women reporting use during 2003–2006. · Use of supplemental calcium increased from 28% during 1988–1994 to 61% during 2003–2006 among women aged 60 and over.
Status of Operations; Consumer product development and sales
More than 150 million Americans drink tea every day, amounting to some 79 billion servings of tea in America every year. Our launch of ViPova black tea brand is meant to tap into this existing demand. Part of our corporate strategy is to build national brands through products that large groups of potential customers are already familiar and comfortable with.
PoViva Tea, LLC (now Poviva Corp.). has filed multiple patents pending and has received several granted patents to bind active hemp oil ingredients with a lipid, potentially allowing for more efficient and comforting delivery of CBD.
Lexaria began producing cash flows from its products in January 2015 focusing on the immediate opportunities in the hemp-oil-sectors that are federally legal. Cannabinoids have been found by many researchers to have antioxidant properties and Lexaria plans to use the patented DehydraTECH process to infuse hemp oils into a number of popular food and beverages.
Lexaria has launched a line of premium products, always relying on its DehydraTECH patented infusion process, to bring hemp oil into the mainstream. Because hemp oil does not have psychoactive properties we expect our products to appeal to the widest possible customer base. To date we have focused our sales efforts across the continental USA. Some studies have found that 3% of the Canadian population regularly consumes hemp food products, while 1% of the American population regularly consumes hemp food products. We believe the consumption of hemp-based food products offers exceptional growth possibilities.
According to Nutrition Business Journal, the Organic Food sector was a $246 billion industry in the USA during 2014, while Dietary Supplements was a $34.6 billion industry. According to Arcview, Legal Cannabis was a $4.7 billion US industry in 2015 and expected to grow to over a $20 billion sector before 2025 but is clearly a much smaller industry sector than the more established food sectors. Lexaria has not yet determined whether our hemp oil-infused products will be accepted into any or all three of these particular sectors.
Lexaria has a main corporate website (www.lexariabioscience.com) as well as smaller e-commerce focused websites devoted to consumer products. The majority of product sales have taken place through the e-commerce websites. A contracted national distribution center ensures rapid and accurate fulfillment of all orders. A 1-800 ordering center has also been placed into operation. Through its subsidiaries, Lexaria currently sells small quantities of 3 flavors of tea, a capsule, and a powdered drink additive, all infused with Lexaria’s technology to deliver multi spectrum hemp oil ingredients.
On June 11, 2015, Lexaria initiated the simultaneous filing of a U.S. utility patent application and an International patent application under the Patent Cooperation Treaty (PCT) procedure, both through the U.S. Patent and Trademark Office (“USPTO”). These applications follow the Company’s 2014 and 2015 family of provisional patent application filings in the U.S. and serve two additional broad purposes:
| 1. | Lexaria is seeking protection of its intellectual property under international treaties. To this end Lexaria has filed for PCT patent application protection. There are 148 countries that are signatories to the Patent Cooperation Treaty, including such major markets as Canada, China, India, much of Europe and the Middle East, the United Kingdom and Japan among others. |

| Page 10 of 83 |
| Table of Contents |
| 2. | Lexaria believes its lipid infusion technology has applications beyond the delivery of just cannabinoids. Based on further formulation testing, Lexaria has included additional lipophilic molecules that may be delivered via food and beverage formats utilizing its technology, widely encompassing three major new market opportunities for the Company: Nicotine; Nonsteroidal Anti-Inflammatories (NSAIDs); and Vitamins. |
In December 2015, the Company filed two further provisional patent applications in the U.S. These new applications served to further broaden the variety and applicability of base compounds that can be used when formulating the Company’s lipid-based technology. The first of these applications identify compounds like edible starches (e.g., tapioca starch) that are commonly used in food products today and could, therefore, serve as a base for formulating and incorporating the Company’s Technology into a wide variety of every day food products. The second of these applications identify emulsifier compounds like gum arabic that are commonly used in beverage products today in order to facilitate similar flexibility for formulating the Company’s technology in every day, shelf-stable beverages.
On October 26, 2016, the USPTO issued U.S Patent No. 9474725, Cannabinoid Infused Food and Beverage Compositions and Methods of Use Thereof, pertaining to our method of improving bioavailability and taste of certain cannabinoid lipophilic active agents in food products. This was the Company’s first patent granted and has a publish date of October 27, 2016 (June 15 2017 in Australia No. 2015274698) and protects our technology for twenty years. Additional patent grants include, but are not limited to: the use of DehydraTECH technology as a delivery platform, “composition of matter” claims that protect the specific combination of substances which enable improved taste and bio absorption properties, that protect processes for making specific compositions of matter for enhanced cannabinoid delivery utilizing the DehydraTECH technology. Of note, Lexaria has received issuance of patents in its second and third patent families representing the first time the Company has been granted claims for use of its technology in connection with the treatment of specific diseases and medical conditions affecting humans, which the Company believes will prove to be of significance to the pharmaceutical industry sector as it further develops and grows. Our portfolio consists of the following granted patents:
Issued Patent # | Patent Issuance Date | Patent Family |
US 9,474,725 B1 | 10/25/2016 | Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof
|
US 9,839,612 B2 | 12/12/2017 | |
US 9,972,680 B2 | 5/15/2018 | |
US 9,974,739 B2 | 5/22/2018 | |
US 10,084,044 B2 | 9/25/2018 | |
US 10,103,225 B2 | 10/16/2017 | |
US 10,381,440 | 8/13/19 | |
US 10,374,036 | 8/06/19 | |
AUS 2015274698 | 6/15/2017 | |
AUS 2017203054 | 8/30/2018 | |
AUS 2018202562 | 8/30/2018 | |
AUS 2018202583 | 8/30/2018 | |
AUS 2018202584 | 1/10/2019 | |
AUS 2018220067 | 7/30/19 | |
AUS 2016367036 | 7/30/19 | Methods for Formulating Orally Ingestible Compositions Comprising Lipophilic Active Agents |
AUS 2016367037 | 8/15/19 | Stable Ready-to-Drink Beverage Compositions Comprising Lipophilic Active Agents |
The Company does not know and cannot know whether these strategies will be successful, or if successful, how long it will take to gain consumer acceptance and customer loyalty. It can be a challenge to be successful by introducing new consumer products to a competitive retail marketplace, and we can offer no assurances that our products will be a commercial success.

| Page 11 of 83 |
| Table of Contents |
International Patent Protection
When Lexaria first began examining the legal medical cannabis market in 2013, and entered the market in 2014, the Company believed it could make an impact in perhaps both the Canadian and U.S. marketplaces. Our pursuit and development of technology has expanded our potential area of impact, both geographically and by sector. Because of the applicability of our technology to markets outside of the legal cannabis sector, we have taken the necessary steps to protect that intellectual property within larger global markets in other unrelated sectors such as nicotine, vitamins, and pharmaceuticals.
Additional Molecules
NICOTINE. More than 99% of all nicotine that is consumed worldwide is delivered through smoking cigarettes. Approximately 6,000,000 deaths per year, worldwide, are attributed primarily to the delivery of nicotine through the act of smoking according to the Centers for Disease Control and Prevention, which also estimates that over $170 billion per year is spent just in the USA on direct medical care costs for adult smokers. 69% of U.S. adult smokers want to quit smoking and 43% of US adult smokers have attempted to quit in any twelve-month period.
Worldwide, retail cigarette sales were worth $722 billion in 2013, with over 5.7 trillion cigarettes sold to more than 1 billion smokers.
NON-STEROIDAL ANTI-INFLAMMATORIES. NSAIDs are the second-largest category of pain management treatment options in the world. The global pain management market was estimated at $22 billion in 2011, with $5.4 billion of this market being served by NSAIDs. The U.S. makes up over one-half of the global market. The opioids market (such as morphine) form the largest single pain management sector but are known to be associated with serious dependence and tolerance issues.
Some of the most commonly known NSAIDs are ASA (Aspirin), Ibuprofen (Advil, Motrin), and Acetaminophen (Tylenol - Acetaminophen is not accepted by all persons to be an NSAID). Although NSAIDs are generally a safe and effective treatment method for pain, they have been associated with a number of gastrointestinal problems including dyspepsia and gastric bleeding.
VITAMINS. The global vitamin and supplement market is worth $68 billion according to Euromonitor. The category is both broad and deep, comprised of many popular and some lesser known substances. Vitamins in general are thought to be an $8.5 billion annual market in the U.S. The U.S. is the largest single national market in the world, and China and Japan are the 2nd and 3rd largest vitamin markets.
Vitamin E is fat soluble and can be incorporated into cell membranes which can protect them from oxidative damage. Global consumption of natural source vitamin E was 10,900 metric tons in 2013 worth $611.9 million.
On August 11, 2015, Lexaria signed a license agreement with PoViva Tea LLC for $10,000, granting Lexaria a 35-year non-exclusive worldwide license to unencumbered use of PoViva Tea LLC’s IP Rights, including rights of resale. This license agreement ensures Lexaria has full access to the underlying infusion technology. On January 14, 2019 this agreement was updated whereby Poviva Corp. granted Lexaria an exclusive license to the DehydraTECH technologies lasting the later of 25 years of the expiration date of the last of Poviva Corp.’s granted patents.
Scientific testing and validation
On August 24, 2015, the Company announced potential industry-changing achievements in enhanced gastro-intestinal absorption of CBD utilizing Lexaria’s technology. The third-party testing was conducted in two phases of in vitro tests beginning in June and completed in August 2015.
The independent laboratory results delivered average CBD permeability of 499% of baseline permeability, compared to CBD permeability without Lexaria’s Technology. These results exceed Company expectations. This was assessed in a strictly controlled, in vitro experiment using a human intestinal tissue model. Samples of Lexaria’s commercially available CBD-fortified ViPova black tea were administered in the model compared with concentration-matched CBD control preparations that lacked Lexaria’s patented formulation and process enhancements. Lexaria believes that its in vitro findings provide compelling evidence of the intestinal absorption enhancing capabilities of its technology, based on which it is exploring opportunities to progress to more advanced, follow-on bioavailability testing in animals.

| Page 12 of 83 |
| Table of Contents |
The tests also showed 325% of baseline gastro-intestinal permeability of CBD comparing Lexaria’s CBD-fortified ViPova black tea to a second control of CBD and black tea combined, without Lexaria’s patented formulation enhancements. This confirmed that the specialized processing undertaken by Lexaria during its manufacturing process together with its formulation enhancements, does indeed significantly improve absorption levels.
The bioavailability of CBD (or of THC) varies greatly by delivery method. Smoking typically delivers cannabinoids at an average bioavailability rate of 30% (Huestis (2007) Chem. Biodiverse. 4:1770–1804; McGilveray (2005) Pain Res. Manag. 10 Suppl. A:15A – 22A). By comparison, orally consumed cannabis edibles typically deliver cannabinoids at an average bioavailability rate of only 5% (Karschner et al. (2011) Clin. Chem. 57:66–75).
The Company’s present findings suggest that its technology may achieve a 5-fold improvement in cannabinoid absorption in edible form over that which can be achieved without its proprietary process and formulation enhancements. This conceptually supports that Lexaria’s technology represents a significant breakthrough in cannabinoid delivery by approximating the high absorption levels achieved as though through administration by smoking, but without the associated negative effects on human health caused by smoking.
The tests were completed in two phases culminating with testing using simulated intestinal fluid conditions that delivered these findings. These results were stronger than earlier iterations of the tests that did not use a simulated intestinal fluid environment and contributed to Lexaria’s understanding of the mechanisms at work. For these and other reasons, Lexaria believes that bioavailability testing in animals is likely to yield even stronger absorption results in the presence of natural intestinal fluid conditions.
CBD has been repeatedly found to provide beneficial pain relieving, anti-inflammatory, anti-anxiety, neuroprotection, anti-psychotic, and anti-convulsive effects among others. Lexaria’s patented technology could significantly reduce individual serving requirements for CBD to consumers. This could lead to reduced costs of consumption for consumers and increased profitability for Lexaria.
Lexaria believes that the same technology used to enhance the absorption of CBD in the recent laboratory tests, is applicable to THC, nicotine, NSAIDs and other lipophilic compounds that are widely used today.
During January 2015, Lexaria conducted a study of nitric oxide levels in humans, as a biomarker for absorption of CBD, with the expectation that it would provide additional evidence of the efficient absorption of CBD from Lexaria food products enhanced with hemp oil, by demonstrating the elevation of nitric oxide in the human body in response to product ingestion.
The study data from human subjects demonstrated significant elevation of systemic nitric oxide levels as a surrogate biomarker for CBD bio absorption in response to ingestion of Lexaria’s products. This provided clinical support for the CBD bioavailability enhancing properties of Lexaria’s patented Technology, on the premise that bioavailable CBD is known to elevate levels of the endocannabinoid anandamide in the human body which, in turn, stimulates release of nitric oxide in the vascular system.
In summary, consuming Lexaria’s food products resulted in elevated levels of nitric oxide within the body. The results of the study indicated that all of Lexaria food products elicited significant increases in salivary nitric oxide, achieving levels from 110 µM to as high as 220 µM in the test subjects. The ViPova beverage products generally had faster initial responses in as little as 15 minutes after product ingestion, whereas the initial responses from the Lexaria Energy protein-energy bars required 30 minutes. The faster response time with the beverage products was to be expected, given the relative ease of digesting liquids versus solids. All products sustained their maximum levels of nitric oxide detection through to the 60-minute end-points used in the study, indicating a need for additional study to determine the length of time that nitric oxide levels remain elevated following production consumption.
The study assessed six flavors of ViPova tea (Yunan Black, Herbal Cherry Black, Earl Grey, Herbal Bengal Chai, Herbal Masala Chai and Decaf English Breakfast), ViPova Columbian Supremo Coffee, ViPova Hot Chocolate and Lexaria Energy Foods’ Chocolate Berry Date and Cashew Berry Date protein-energy bars.
Six healthy human subjects (3 male and 3 female) between the ages of 22 and 65 years of age were recruited for the study. Subjects were screened for cardiovascular and allergic response to hemp products, were non-smokers and did not have any history of substance or alcohol abuse. One product was studied per day across all six subjects, with each subject consuming a full product serving size. Subjects were required to refrain from eating food or using vape products for at least 12 hours before test article administration on each day of the study. Nitric oxide levels in the test subjects were assessed using a commercially available, colorimetric test kit designed to quantify systemic nitric oxide via a detectable salivary marker. Immediately before test article administration each day, all subjects were required to demonstrate a negative baseline nitric oxide saliva test. Subjects were considered to have a negative test strip reading at a level of 20 µM according to the test strip scale, and positive readings anywhere above this. Subjects performed salivary nitric oxide testing at 15, 30, 45 and 60 minutes’ post-consumption of each product. All subjects remained sedentary from baseline through to the completion of testing for each product.

| Page 13 of 83 |
| Table of Contents |
In August of 2018 we released results from our TurboCBD capsules in a randomized, placebo-controlled, double-blind European human clinical study that evaluated TurboCBD - a proprietary, DehydraTECH powered, CBD fortified hemp oil capsule developed by Lexaria. The degree and speed of CBD absorption into blood plasma and potential cardiovascular and cognitive performance enhancement in 12 healthy male volunteers were studied.
Key bioavailability data highlights from the study comparing the 90 mg dose of Lexaria’s TurboCBD to a 90 mg dose of a positive control formulation without Lexaria’s DehydraTECH technology were as follows:
· 30 Minutes: CBD delivered from Lexaria’s TurboCBD capsules was absorbed much more effectively than from the positive control, delivering 317% more CBD to blood at the 30-minute mark of the study (i.e., 18.4 ng/mL compared to only 4.4 ng/mL on average respectively [95% CI; p=0.051]); · 60 Minutes: The TurboCBD capsules went on to deliver more CBD to the blood at the 60-minute mark (i.e., 38.8 ng/mL) than the positive control capsules were able to reach at any time during the 6-hour study, further demonstrating the exceptional rapidity of action and effectiveness of the TurboCBD capsules; · 90 Minutes: The TurboCBD capsules further went on to deliver significantly more CBD to the blood (86% more) than the positive control capsules at the 90-minute mark (i.e., 53.0 ng/mL compared to only 28.4 ng/mL respectively [95% CI; p=0.034]); · Through to Study Completion: Lexaria’s TurboCBD capsules continued to deliver more CBD to blood than the positive control capsules at each subsequent time point in the study through to the 6-hour mark when the study was completed.
Additional study analysis was released in February 2019:
Key metabolic and hemodynamic performance findings linked to bioavailability enhancements were revealed in the study, which compared a 90 mg dose of Lexaria’s TurboCBD to a 90 mg dose without Lexaria’s DehydraTECH™ technology (the “positive control”) as well as a placebo, as follows:
· Analysis of mean arterial blood pressure (MAP) at peak blood levels of CBD achieved with Lexaria’s TurboCBD demonstrated a significant reduction in MAP compared to placebo (95% CI; p=0.027). This finding was not observed with the dose-matched positive control formulation for which there was no significant decrease in MAP compared to placebo (95% CI; p=0.625); · Cerebral perfusion was also analysed by an index of conductance in the middle cerebral artery (MCA). The findings revealed that Lexaria’s TurboCBD caused the greatest increase in MCA conductance relative to both the positive control formulation and placebo (95% CI; p=0.017 and P=0.002 respectively);
Finally, over the six-hour study, analysis of the total area under the curve (AUC) demonstrated that Lexaria’s TurboCBD resulted in a notable trend for higher levels of CBD in the bloodstream overall than the positive control formulation with total AUC of 10,865 ± 6,322 observed with Lexaria’s formulation compared to 7,115 ± 2,978 observed with the positive control (95% CI; p=0.096). Furthermore, when normalized to body mass, the AUC at the peak CBD concentration was markedly and significantly (95% CI; p=0.02) higher with the TurboCBD 90 mg dose compared to the 90 mg dose positive control formulation.
These results corroborate and confirm other in vitro and in vivo studies that have evaluated Lexaria’s DehydraTECH technology. Although this study evaluated absorption only of CBD and its metabolites, Lexaria believes nearly identical bioavailability enhancement results would be achieved with other cannabinoids.
During March of 2019 we also launched an in vivo research program to test Lexaria designed nanotech enhancements comprised of eleven separate animal studies and released initial results during May 2019 demonstrating measurable quantities of cannabidiol into blood in as little as 2 minutes. In each arm of the animal studies, 10 male Sprague-Dawley rats were administered CBD at 25mg per kg of bodyweight. Delivery of CBD into the bloodstream was monitored over a 60-minute duration. In the first animal study results, Lexaria compared its standard DehydraTECH formulation that combines cannabinoids with long-chain fatty acids (“LCFA”) using Lexaria’s patented dehydration processing technique to a concentration-matched formulation utilizing coconut oil which is a commonly used MCT oil in the cannabis edibles industry.
| · | At 2 minutes DehydraTECH’s LCFA formulation delivered measurable CBD in blood, compared to no measurable CBD in blood until 6 minutes and onwards for the MCT oil formulation. |
|
|
|
| · | At 15 minutes DehydraTECH’s LCFA formulation achieved a CBD blood concentration level that was 475% more than the MCT oil formulation; and, the DehydraTECH LCFA formulation CBD blood levels reached at 15 minutes were greater than the CBD blood levels reached by the MCT oil formulation at any time point during the 60-minute evaluation. |

| Page 14 of 83 |
| Table of Contents |
· At 60 minutes DehydraTECH’s LCFA formulation achieved a CBD blood concentration level of 319% more than the MCT oil formulation. · Over the entire 60-minute study, the animals that received the standard DehydraTECH LCFA formulation achieved an average maximum CBD blood concentration level that was 334% more than the average maximum blood concentration level of the animals that received the MCT oil formulation (p<0.0021). · Over the entire 60-minute study, the area under the curve (AUC) (total quantity of CBD delivered) for the Lexaria DehydraTECH LCFA formulation was 389% more than the MCT oil formulation (p<0.0011).
Lexaria also tested for brain tissue concentrations to quantify 8-hour CBD delivery from the DehydraTECH-enabled LCFA formulation compared to the MCT oil formulation and DehydraTECH’s LCFA formulation outperformed the MCT oil formulation by 246%.
The Company released additional results from its March 2019 research program wherein animal testing proved that combining Lexaria’s DehydraTECH delivery technology with generic nanotech techniques delivers 1,137% more CBD into animal brain tissue following oral ingestion than certain existing industry formulations. Lexaria combined its DehydraTECH delivery technology with a standard form of nanotechnology and analyzed subsequent delivery into brain tissue following oral ingestion. Delivery of CBD into the brain was reported 8 hours after dosing.
· The Lexaria DehydraTECH LCFA formulation without nanotech achieved an average brain tissue accumulation level that was 246% higher than the average for those animals that received the MCT oil formulation (p=0.0013). · The Lexaria DehydraTECH LCFA formulation with nanotech achieved an average brain tissue accumulation level that was 1,137% higher than the average for those animals that received the MCT oil formulation (p=0.0178).
Further results demonstrated that Enhanced DehydraTECH led to 811% more CBD delivery into blood than generic industry MCT coconut-oil formulations (p=0.00008); and 110% more CBD into blood than DehydraTECH in its traditional format (p=0.02).

| Page 15 of 83 |
| Table of Contents |
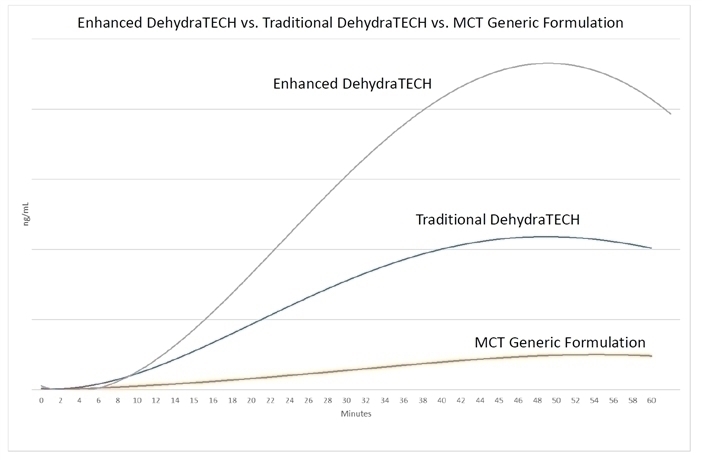
| · | Enhanced DehydraTECH delivered roughly twice as much CBD to animal blood at all measured time points in the study from the 15-minute mark onwards, compared to traditional DehydraTECH; and during the same time points from 717% to 1098% more CBD than the generic industry MCT coconut oil formulations. |
|
|
|
| · | Enhanced DehydraTECH delivered more CBD to blood in just 12 minutes than the MCT coconut-oil formulation was able to achieve at any point during the 1-hour test duration. |
|
|
|
| · | Enhanced DehydraTECH is even faster acting, reaching a maximum blood concentration level (“tmax”) in just 45 minutes compared to traditional DehydraTECH at 50 minutes and the MCT coconut oil formulation at 57 minutes. |
|
|
|
| · | Enhanced DehydraTECH delivered an astonishing 1,937% more CBD into animal brain tissue after 8 hours compared to generic industry MCT coconut oil formulations; and 487% more than traditional DehydraTECH. |
Both traditional DehydraTECH and Enhanced DehydraTECH delivered maximum blood concentration levels prior to the 60-minute end-of-test, with levels tapering off thereafter. The DehydraTECH technology therefore demonstrates both fast onset and fast offset as tested which is of interest for dose titration purposes when repeated dosing is desired.
We have also completed our first study evaluating DehydraTECH used in a topical cream formulation for absorption of CBD through human skin. Results proved significant increases in both speed and quantity of CBD absorption through skin when compared to control formulations. The absorption study was performed on human skin at a California-based laboratory that specializes in Franz diffusion cell skin permeability testing. Lexaria’s DehydraTECH technology was used together with a sophisticated oil-in-water emulsion formulation design and compared to a series of matching oil-in-water emulsion formulations prepared with the same CBD inputs, with and without the DehydraTECH technology and with and without two leading skin penetration enhancers currently used in the skin products industry. Several factors were measured, including the time required to detect CBD skin penetration and quantity, and peak amounts of CBD absorbed into and through the skin, at multiple testing intervals over a 48-hour duration.

| Page 16 of 83 |
| Table of Contents |
Lexaria’s DehydraTECH-enabled topical formulation, absent either of the commercial penetration enhancers, was the fastest acting for absorption into the epidermis, dermis or through the skin into the systemic fraction representing permeation into the underlying circulatory system. Lexaria’s DehydraTECH-enabled product also had no odour even without the use of perfumes, contrary to other cannabinoid industry products that can be quite strongly odoriferous without the use of masking perfumes.
Furthermore, Lexaria’s DehydraTECH-enabled topical formulation without the addition of either of the commercial penetration enhancers, demonstrated the highest overall average quantity of CBD delivered through the skin and into the representative systemic fraction of all the formulations tested, with as much as a 225% increase in CBD permeability when compared to the highest performing commercial penetration enhancer formulation assessed and almost a 1,900% increase in CBD permeability when compared to a control formulation that was devoid of both the DehydraTECH technology or any commercial penetration enhancers. The commercial skin penetration enhancers only demonstrated performance that was on par or superior to the DehydraTECH-enabled formulations tested in so far as total CBD absorption into the shallow epidermis or dermis was concerned.
We have also completed our first ingestible nicotine in vivo (animal) absorption study. Lexaria is pursuing the use of its patented DehydraTECH technology as a possible new nicotine delivery method, an edible dose absorbed through the gastrointestinal tract, with potential both as a nicotine replacement therapy as well as an alternative product format for regular tobacco users.
DehydraTECH delivered the following major nicotine absorption performance improvements: 1,160% faster delivery of equivalent peak quantities of nicotine to the bloodstream than achieved with controls (within 15 min vs. 2.9 hours), 148% gain in the quantity of peak nicotine delivery to the bloodstream relative to controls, 560% higher brain levels of nicotine where nicotine effects are focused, compared to controls, Lower urine levels of nicotine excreted than controls, for enhanced nicotine activity and bioavailability over the course of the study, lower quantities of key liver metabolites in the bloodstream than controls as hypothesized, suggesting bypass of first pass liver metabolism.
Study Design Parameters:
The study was designed to principally assess the relative ingestible nicotine absorption performance of DehydraTECH-powered formulations compared to concentration-matched control formulations that lacked any form of delivery enabling technology in rats. Nicotine was administered in a nicotine polacrilex derivative format as is widely commercialized today in nicotine replacement therapy products such as chewing gums. Twelve male rats were divided into four groups of three, such that DehydraTECH and control formulations were each tested at a 1 mg/Kg and 10 mg/Kg dosage level. Formulations were administered orally and all rats were cannulated for blood collection at multiple intervals over an 8 hour duration post-dosing with the first data collection at the 15-minute mark. Urine and feces were also collected for up to a 24-hour duration post-dosing, and essential organ tissue samples were also collected for examination after the study. All samples were subjected to analytical testing in order to quantify the levels of nicotine therein, as well as the levels of three major liver metabolites thereof, hydroxycotinine, nicotine N’-oxide and cotinine, in order to assess the relative metabolite levels absorbed by the different formulations. Lexaria’s hypothesis was tested to prove that its DehydraTECH technology would influence more rapid and complete intestinal bio absorption of nicotine lymphatically with less metabolic degradation by the liver. All animals were also assessed for general tolerability of the administered formulations. The study was conducted at the same independent laboratory in Philadelphia where the Company completed its initial CBD absorption study in 2015.
Results & Observations:
The Lexaria formulations generally achieved faster absorption, higher peak absorption and higher overall quantities of nicotine, on average, in the blood than the concentration-matched control formulations at both the 1mg and 10 mg/Kg doses tested. Furthermore, as previously reported, there were no obvious signs of gastrointestinal distress such as vomiting or diarrhea indicating that the animals appeared to tolerate the treatment well.
Nicotine blood levels were evaluated multiple times over a period of 8 hours after dosing. In the 10mg/Kg dosing arm, the control formulation required nearly 3 hours to reach similar levels of blood absorption that the Lexaria formulation reached in only 15 minutes. Furthermore, the Lexaria formulation went on thereafter to demonstrate peak plasma levels that were 148% of those achieved by the control formulation. If replicated in human studies, these findings are suggestive that Lexaria’s technology could prove more effective in elevating blood nicotine levels through edible formats much more quickly and substantially than previously theorized, potentially making ingestible nicotine preparations a viable alternative to today’s available product formats while also leading to a more rapid nicotine craving satiation.

| Page 17 of 83 |
| Table of Contents |
Analysis of the liver metabolites revealed, as expected, that overall levels in the blood of two of the three metabolites studied were higher in the control group than in the Lexaria formulation group at the 10 mg/Kg dose. This result was especially pronounced in the 45-minute to 2-hour time interval post-dosing which is consistent with the expected timing of release of metabolites in higher quantity into the bloodstream by the liver following normal physiological processing of ingested nicotine with the control preparation, compared to the DehydraTECH technology that is believed to elude first pass liver metabolism. The Lexaria formulation also demonstrated lower quantities of nicotine in the rat urine at both doses, which is consistent with the fact that the levels of nicotine in the rat blood remained higher over the duration of the study with the Lexaria formulation than with the control. The study also revealed that the Lexaria formulation at the 10 mg/Kg level achieved up to 5.6-times as much nicotine upon analysis of the rat brain tissue than was recovered with the matching control formulation. These findings together perhaps suggest prolongation of nicotine effectiveness with the Lexaria formulation which may also be beneficial in humans to control cravings over an extended time-period from a single edible nicotine dose.
In our follow-up third-party in vivo statistically significant study, including two groups of 20 animals, further defining delivery of nicotine in edible form at each of the 2, 4, 6, 8 and 10-minute intervals post-dosing, with 90.2% greater delivery than the concentration-matched control formulation by the 10-minute mark (95% CI; p=0.044), and significantly greater absorption levels than the control formulation at all subsequent time points in the study. Speed of onset is a key attribute for oral drug administration, and it is of particular importance for the consideration of non-inhalation nicotine delivery formats.
Key highlights of the follow-up study are as follows:
· Peak Level: 79% improvement in peak blood levels (maximum concentration or “Cmax”) at 394 ng/mL using Lexaria’s DehydraTECH technology vs. 220 ng/mL with the control (95% CI; p=0.0257); · Total Quantity: 94% improvement in total quantity of nicotine delivered (area under the curve or “AUC”) to the blood during the 60-minute course of the study, at 266 hr•ng/mL versus 137 hr•ng/mL (95% CI; p=0.0086); · Rapidity: Lexaria’s technology delivered nicotine into the blood stream by the first time interval of blood sampling at the 2-minute mark. On average, Lexaria’s technology delivered 203 ng/mL to the blood in aggregate of the 2, 4, 6, 8, 10, 12 and 15-minute time points, compared to only 120 ng/mL in aggregate over the same period by the control, an improvement of 70% (95% CI; p=0.0004).
In addition to the above described scientific testing and validation studies, Lexaria has also conducted various cannabinoid formulation experiments, together with potential DehydraTECH licensee partners, on chocolates, candies, gummies, mouth-melts, chocolate bars, protein bars, beverages such as beer, spices, tea, coffee, supplements and more over the past several years. Beverage formulations have produced cannabinoid water-based products including de-alcoholized beer that mask unwanted cannabis flavor and are fast acting. Chocolate formulations were reported as being the fastest acting, most consistent, and best-tasting products relative to comparator control formulations in approximately 70% of cases in a recent 2017 consumer study. As well, on March 22, 2016, Lexaria announced results from another chocolate formulation consumer study in which test subjects ranked those chocolates that had been created with Lexaria’s technology as the best tasting, most palatable and providing the best overall experience of the chocolates sampled. Furthermore, the test subjects in that study indicated a time of onset of the cannabis oil effects in as little as 15-20 minutes on average. The study included 12 volunteers who were all regular cannabis consumers with experience ingesting conventional edibles. All chocolates used in the study were blinded (unmarked) in order that the subjects could not discern the product formulations applied.
Technology out-licensing
On May 14, 2016, the Company entered into a Licensing Agreement with Nuka Enterprises, LLC (“Nuka”) for a two-year period, to utilize the Company’s technology to create, test, manufacture, and sell marijuana-infused consumable and/or topical products, in the state of Colorado, with an option of extending the terms of the Licensing Agreement to Washington, Oregon, and California. On April 30, 2018, the Company announced a new 10-year renewal licensing agreement with Nuka, maker of 1906 brand cannabis chocolates and other edible products. The new agreement provides Nuka with semi-exclusive ability to utilize the DehydraTECH technology across the US. Nuka also acquired an option to expand its products and brand to Canada, including using Lexaria’s existing chocolate and confections contract manufacturer licensee Cannfections Group Inc. The agreement incorporates new rights in product categories in addition to the original chocolate formats, which include candies, beverages, capsules and pills, and topical creams. On May 21, 2019, we announced a major expansion in operations by Nuka over the next two years into Illinois, Ohio, Massachusetts, Michigan and other states. The comprehensive semi-exclusive agreement provides Nuka and 1906 with competitive technological advantages until 2028. A second license provides Nuka and 1906 with the immediate ability to utilize DehydraTECH technology for CBD across the US marketplace.

| Page 18 of 83 |
| Table of Contents |
On January 25, 2018, the Company announced it entered a definitive technology licensing agreement with a 7-year term with Cannfections Group Inc. whereby Lexaria is providing its patented DehydraTECH technology to empower next-generation performance in cannabis infused chocolates and candies to be developed and sold in Canada and internationally.
On February 26, 2018 the Company announced it entered an agreement with NeutriSci International Inc. whereby Lexaria granted an Intellectual Property License and Supply Agreement for the manufacturing and sale of CBD-based products. This agreement has been terminated effective March 15, 2019.
On February 27, 2018 the Company announced it entered a definitive technology licensing agreement with Los Angeles-based, privately-held Biolog, Inc. (“Biolog”) for a 5-year term whereby Lexaria provided its patented DehydraTECH technology to empower a unique set of next-generation food and beverage cannabis infusion products to be sold in the United States. On June 10, 2019 the Company terminated its license with Biolog.
On April 25, 2018, the Company announced that it entered a definitive technology licensing agreement with GP Holdings LLC, (“GP”) whereby Lexaria provided its patented DehydraTECH technology for cannabis infused beverages and topical skin products in California. GP acquired a 5-year semi-exclusive right. Subsequent to year end, on September 28, 2018, the Company cancelled the contract due to ongoing delays and non-performance.
On July 31, 2018, the Company and Hill Street Beverage Company Inc., (TSXV:BEER; “Hill Street”) jointly announced that they signed a Definitive Agreement to license Lexaria’s DehydraTECH, on a semi-exclusive basis, for a term of five (5) years, to produce a line of cannabis-infused alcohol-free beverages for Canadian distribution, following regulatory approval.
On January 15, 2019, the Company announced that its wholly-owned subsidiary Lexaria Nicotine and Altria Ventures Inc., an indirect wholly owned subsidiary of Altria Group, Inc. (“Altria”), executed definitive agreements to pursue innovation in oral, reduced risk nicotine consumer products using Lexaria’s patented DehydraTECH technology. Altria was granted a license to use Lexaria’s DehydraTECH technology for oral nicotine delivery forms on an exclusive basis in the United States and a non-exclusive basis elsewhere globally. Altria will pay Lexaria Nicotine a royalty on revenue generated from the sale of nicotine products containing DehydraTECH, until such time it may acquire 100% ownership in Lexaria Nicotine. There is no requirement that Altria must acquire 100% ownership in Lexaria Nicotine.
On April 24, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Canpharm ULC, to provide Lexaria’s patented DehydraTECH technology to a private California-based company for its utilization in certain CBD-based beverages to be produced and sold in California and Nevada that may include any combination of ready-to-drink beverages such as non-alcoholic beers, wines and spirits; cold or hot coffee or teas, sports drinks and more.
On May 7, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Hemp Corp., to provide Lexaria’s patented DehydraTECH technology to a private Nevada-based company for its utilization in certain CBD-based beverages to be produced and sold across the USA that may include any combination of ready-to-drink beverages such as non-alcoholic beers, wines and spirits; cold or hot coffee or teas, sports drinks and more.
On May 21, 2019, the Company announced a major expansion in operations by Nuka and its 1906 brand of edibles over the next two years into Illinois, Ohio, Massachusetts, Michigan and other states. The comprehensive semi-exclusive agreement provides Nuka and 1906 with competitive technological advantages until 2028. A second license provides Nuka and 1906 with the immediate ability to utilize DehydraTECH technology for CBD across the US marketplace.
On July 10, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Hemp Corp., to provide Lexaria’s DehydraTECH technology to Nic’s Beverages Ltd for use in CBD-based beverages to be produced and sold throughout the United States.
On July 11, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Hemp Corp., to provide Lexaria’s DehydraTECH technology to Universal Hemp LLC, a B2B manufacturing company of hemp-derived bulk ingredients to the nutraceutical and consumer packaged goods industries to be produced and sold across the USA immediately, and in Canada when regulations permit. Agreed to minimum payments over the life of the 5-year agreement are $3,750,000.

| Page 19 of 83 |
| Table of Contents |
On July 24, 2019, the Company announced that it entered a 10-year Joint Manufacturing Partnership (JMP) with Hill Street to produce commercial products including processed THC cannabis and/or CBD hemp powder including among other categories; tablets, capsules, or packets for sale in Canada and for export where permitted. The JMP will also produce similar powders as a bulk ingredient for manufacturing processes for sale to other licensed producers seeking to use DehydraTECH to create their own products for sale within Canada. Profits from this business unit will be shared equally between Hill Street and Lexaria. In addition to the JMP, Hill Street acquired two global semi-exclusive licenses (with minor exceptions) to utilize Lexaria’s DehydraTECH THC beverage infusion technology around the world, valid for 10 years. Under the terms of the agreement, Hill Street will pay an annual licensing fee of $15,000 and up to $1,800,000 to Lexaria by issuing $800,000 in common shares of Hill Street to Lexaria initially with up to an additional $500,000 in shares of Hill Street when they enter each of the first two international markets subject to TSXV and CSE approval, as applicable. Pursuant to the terms of the JMP agreements, Lexaria will issue an aggregate $250,000 in restricted common shares to Hill Street. Closing of the Hill Street / Lexaria agreements is subject to normal regulatory approvals and the closing of the Hill Street / OneLeaf transaction announced by Hill Street. Subsequent to August 31, 2019, Hill Street has been unable to close its transaction with OneLeaf and is currently searching for an alternate location from which to base the Hill Street / Lexaria agreements, thus there is a possibility that this transaction may not close as expected.
The continuation of our business interests in these sectors is dependent upon obtaining further financing, a successful program of development, and, ultimately, achieving a profitable level of operations. The issuance of additional equity securities by us could result in a significant dilution in the equity interests of our current stockholders. Obtaining commercial loans, assuming those loans would be available, will increase our liabilities and future cash commitments.
We are not yet profitable and have not yet demonstrated our ability to generate significant revenues from our business plan. We will require additional corporate funds if our existing capital is not sufficient to support the Company until potential future profitability is reached. There are no assurances that we will be able to obtain further funds required for our long-term operations. We expect to require additional operating capital during our fiscal 2020 year. There can be no assurance that additional financing will be available to us when needed or, if available, that it can be obtained on commercially reasonable terms. If we are not able to obtain the additional financing on a timely basis, we will be unable to conduct our operations as planned, and we will not be able to meet our other longer-term obligations as they become due. In such event, we could be forced to scale down or perhaps even cease our operations. There is uncertainty as to whether we can obtain additional long-term financing if we do in fact require it.
Our business plan anticipates that we will hire two to four additional staff during fiscal 2020 to enhance operations in our new office and licenced laboratory space. We expect to be able to utilize contracted third-parties for our production and distribution needs, instead focusing our capital on higher value-added aspects of the business such as research and development, and scientific testing. We have no current plans to build our own production facility.
Our company relies on the business experience of our existing management, on the technical abilities of consulting experts, and on the technical and operational abilities of its operating partner companies to evaluate business opportunities.
Competition
Competition in alternative health sectors and in consumer products in the USA is fierce. We expect to encounter competitive threats from existing participants in the sector and new entrants. Although PoViva Corp. has filed patent applications to protect intellectual property, there is no assurance that patents beyond those already issued will be granted nor that other firms may not file superior patents pending. Food supplements, organic foods, and health food markets are all well established and the Company will face many challenges trying to enter these markets. Lexaria is also aware of various competing technologies that exist in the marketplace that claim to also enhance the bio absorption of cannabinoids as Lexaria has demonstrated through repeated in vitro and in vivo scientific testing with its patented DehydraTECH technology. By and large, these technologies are mostly forms of nanotechnology that generally claim to enable the formation of microencapsulated microemulsions of cannabinoid active ingredients. These technologies can enable exceptional water solubility of cannabinoid ingredients and can impart improved intestinal bio absorption as a result.

| Page 20 of 83 |
| Table of Contents |
Competition in nicotine, alternative nicotine delivery and nicotine cessation sectors in the USA is comprised of long-established entities, brands, and new technologies competing to create less harmful options. The sectors are complicated by the significant historical empirical data of older products or technologies versus the more limited published supporting data regarding the effects of new products or technologies. Due to the size of the sectors we expect to encounter competitive threats from existing participants and unknown new entrants. There is no assurance that other technologies already deployed, or in development, will not form the basis of product formats that competitors or consumers choose to utilize. It is also possible that historic delivery methods that have been in use and the familiarity with them may prevent adoption of products utilizing our technology in alternative delivery formats. Competing technologies or products may utilize known delivery formats or entirely new and unforecastable formats. Lexaria has demonstrated through scientific testing with its patented DehydraTECH technology that it delivers nicotine rapidly and effectively through oral delivery. We believe that if we can educate and influence consumers to adopt a food grade edible product format, and if US regulatory bodies authorize such formats, we may be able to offer a competitively successful new product format that utilizes our technology.
The legal marijuana industry is comprised of several sub-sectors and is legal under different guidelines in many states though it remains illegal under most federal laws. Notwithstanding, the overall sector is generally recognized to be one of the fastest growing in the USA, with state-legal revenue of over $8 billion in 2016. Independent projections and publicized reports expect revenue of $20 billion or more in 2020, both as the sector gains in credibility and acceptance, and as more and more states legalize either medical use or adult recreational use; or both. In June of 2019 there were eleven states and one district that had legalized medical and recreational use, and more than twenty-two other states that had legalized medical use. In any fast-growing industry, competition is expected to be both strong and also difficult to evaluate as to the most effective competitive threats. While we are an early adopter within the cannabinoid delivery sector, there are already reports of more than 300 public companies that have claimed to be involved in the sector in some fashion; and an unknown number of private companies. Our current strategies may prove to be ineffective as the sector grows and matures, and if so, we will have to adapt quickly to changing sectoral circumstances. Accordingly, the Company intends to aggressively pursue technology out-licensing opportunities not only within the cannabinoids sector where it is already active, but also across other sectors where its DehydraTECH technology is patent allowed and/or pending, including the opportunities in the vitamin and supplements sector, the pain relief sector and the nicotine products sector.
However, it is Lexaria’s belief that its patented DehydraTECH technology offers a host of benefits beyond what competing technologies can offer, including superior oral palatability, a more appealing and all-natural ingredient compositional profile from a food and beverage formulation perspective, more predictable time of delivery into bloodstream, and superior scalability and cost effectiveness from a manufacturing perspective. Lexaria believes that its DehydraTECH technology is, therefore, significantly distinguished from competing technologies in these respects, with a view to growing the breadth and number of licensees that will adopt its technology for their product offerings going forward. Lexaria believes that these competitive advantages together with its wealth of scientific data showing noteworthy bio absorption enhancements with its DehydraTECH technology constitute a compelling value proposition for its prospective licensees, and it intends to continue to pursue license arrangements not only within the cannabinoids edibles sector where it is already active, but also in the various other bioactive ingredient sectors identified in its issued and pending patent applications.
Compliance with Government Regulation
Over 30 States in the USA have passed some form of legislation related to that state’s permission to grow, cultivate, sell or use marijuana either for medical purposes or for recreational or “adult use” purposes; or both. The various state legislation is not necessarily harmonious with one another. It is most often not legal to transport cannabis-related products across state lines.
Lexaria does not “touch the plant” or manufacture, process or sell cannabis in any location within the USA. Lexaria does conduct research and development on cannabis ingredients legally in Canada, in a federally licensed laboratory in compliance with all federal and local Canadian laws. We comply with US federal law that provides for certain exemptions for agricultural (industrial) hemp and certain by-products to be manufactured and sold in the US. The DehydraTECH technology may have applications within the legal marijuana sector and we may seek to license that technology to companies that have met and comply with state regulations for the sale or distribution of cannabis related products in any particular jurisdiction.

| Page 21 of 83 |
| Table of Contents |
Lexaria’s position is that, just as a telephone company provides communications services, and an electric company provides electrical power, our provision of technological services to a state-legal cannabis company is in compliance with laws and required regulations.
Lexaria’s patented DehydraTECH technology also has applications in completely separate sectors such as vitamins, NSAIDs, and nicotine. We have no products nor operations in any of these sectors today, although we have commenced formulation development for research and validation purposes in each of these areas. We have a formal relationship with the largest cigarette company in the US and are conducting R&D with that company related to the possible development of nicotine oral products. If we enter any of these sectors at any time, we will be exposed to and of necessity will have to comply with, all local, state and federal regulations in each of those sectors. As a result of the possibility of Lexaria being involved in a number of disparate business sectors, compliance with government regulations could require significant resources and expertise from our company.
The US Federal Government passed the 2018 Farm Bill (the “Bill”), in December of 2018, that may have significant impacts on industry segments that we operate and have products in and potentially change some of the regulatory compliance risks that may affect our business. The Bill includes lifting restrictions on advertising, marketing, banking and other financial services as well as allowing interstate commerce for hemp and hemp-derived CBD, removing barriers for intellectual property protections under federal law such as patents and trademarks, as well as several other measures that may positively impact these industry segments overall. The impact the Bill may have on other regulatory bodies and their regulations will require ongoing monitoring to determine the outcome and timing of any revisions.
Enertopia Joint Venture
On May 28, 2014, our Company entered into a joint venture agreement with Enertopia Corp. for a prospective medical marijuana business under the Canadian Marijuana for Medical Purposes Regulations (“MMPR”) for a 49% net ownership interest in the business (Enertopia 51%) utilizing an identified location in Burlington, Ontario (the “Burlington Joint Venture”).
On June 26, 2015, we entered into a definitive agreement with Enertopia Corp. and Shaxon Enterprises Ltd. to sell our 49% interest in the Burlington Joint Venture and the MMPR application number 10MMPR0610. Pursuant to the sale terms of the agreement, we received a non-refundable $4,900 deposit and are entitled to receive up to $735,000 in milestone payments upon the Burlington facility becoming licensed under the MMPR. Notwithstanding the foregoing, the Company does not expect the grant of a production license for the Burlington facility.
Marijuana Production in the United States
In the United States it is still illegal under federal law to grow, cultivate and sell medical or adult use marijuana. However approximately thirty-two states have approved medical marijuana for use and at least ten states have approved adult use regulations. The United States Federal government justice department has released memos that will respect the individual states where strict guidelines are followed and enforced so that the health, safety and security are protected at all times by state authorities but there is no assurance that federal laws will not at any time be more vigorously enforced. If the individual state framework fails to protect the public the Federal government will act in enforcing the controlled substances act of 1970 and the DEA will enforce the federal law.
As at the date of this document, our company has not entered into any prospective or definitive arrangements to produce or distribute marijuana products in the United States and has no intention of engaging in such marijuana related activities in the United States. However, our company continually reviews opportunities and monitors legal and regulatory developments related the medical marijuana sector in both Canada and the United States. We anticipate that we may re-evaluate our participation in the United States medical marijuana sector in the event that medical marijuana production becomes federally sanctioned and, in the meantime, we plan to limit our foray into the marijuana industry to ancillary involvement based on out-licensing of our DehydraTECH technology to state licensed producers.
On November 8, 2016 referendums held in various US states increased those areas in the USA where either medical or recreational use marijuana was state-legal. More than 50% of the US population now lives in a state where either medical or recreational marijuana use is permitted by state law, although it is still banned by US federal laws.

| Page 22 of 83 |
| Table of Contents |
Contractors
We utilize employees, sub-contractors and consultants in the intellectual property development and licensing, and Company operations. We have added four employees during the year and additional research personnel may be added during fiscal 2020 with the granting of our license from Health Canada for the research lab. We primarily engage with consultants to serve our executive needs.
The Company had an agreement with CAB Financial Services Ltd., wholly-owned by Chris Bunka, for a consulting fee of $144,000 per year and has negotiated a 3-year term renewal management contract with Chief Executive Officer Chris Bunka retroactively effective January 1, 2019. The annual compensation payable is CDN$350,000 per year and the following performance incentives.
Performance Incentives
· A performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the board of directors of Lexaria. Compensation equal to 2% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. Certain compensation to be paid upon a change of control excluding certain circumstances and participation in the Company’s approved stock option plans.
The Company appointed Mr. John Docherty as President of Lexaria effective April 15, 2015. The Company had an agreement with Docherty Management Limited, wholly-owned by Mr. John Docherty with compensation of CAD$180,000 plus applicable taxes per year and has negotiated a 3-year term renewal management contract CAD$300,000 per year and the following performance incentives.
· Performance Incentives as defined above.
On July 1, 2018 the Company executed an updated three-year consulting contract with M&E Services Ltd. (M&E), a company wholly-owned by Mr. Allan Spissinger, with monthly compensation of CAD$12,000 including an 8% annual increase superseding the previous CAD$8,000 per month contract that included 200,000 incentive stock options exercisable at $0.37. The Company may pay Mr. Spissinger a bonus from time to time, at its sole discretion. Mr. Spissinger will be entitled to receive additional common stock-based and stock option-based bonuses upon achieving certain milestones during the time of his consultancy with the Company. These milestones are:
Revenue Incentive Milestones (Revenue Incentives “A”)
| · | 100,000 common shares issuable upon the Company achieving non-refundable revenues of $200,000 to any single customer in any consecutive 60-day period for the first 12 months of the contract, plus a further 50,000 common shares issuable upon achieving non-refundable revenues of $200,000 to any single customer in any consecutive 60-day period, during the 13th - 24th months of the contract. If the Company achieves non-refundable revenues of $500,000 in any fiscal quarter, a further 200,000 common shares may be issuable during the first 12 months of the contract and 100,000 common shares during the 13th - 24th months of the contract. |
Intellectual Property Milestones (IP Incentives “C”)
| · | During the term of the agreement, for each provisional patent application substantively devised by M&E and successfully created, written and filed with the US Patent Office for the Company’s technology, M&E will be entitled to an award of up to 100,000 common shares of the Company to a maximum value of $250,000 at the time of issuance; or a cash award not to exceed $10,000 for an idea or concept originally conceived by M&E but more than 80% of the subsequent work, time and expenses paid for by the Company. |
On June 19, 2017, the Company executed a contract with Alex Blanchard Capital as manager for investor relations and communications. The agreement is for six months continuing month to month for CAD$7,500 per month and may be terminated thereafter with one month’s notice. Mr. Blanchard was granted 200,000 warrants exercisable at $0.29 and 300,000 stock options exercisable at $0.295 vesting 100,000 options at 1st – 3rd anniversaries of the contract provided that the contract is not terminated. Mr. Blanchard will be entitled to receive additional common stock-based and stock option-based bonuses upon achieving certain milestones during the time of his consultancy with the Company. These milestones are during the first 12 months after the date of the agreement with Alex Blanchard Capital:

| Page 23 of 83 |
| Table of Contents |
· Revenue Incentives “A” as defined above.
On December 1, 2017, the Company executed a contract with a contractor as office manager and assistant to the CEO and CFO. The agreement is for two years continuing month to month thereafter and may be terminated with one month’s notice for CAD$6,500 per month. The contractor was granted 250,000 warrants exercisable at $0.83. They will be entitled to receive additional common stock-based and stock option based bonuses upon achieving certain milestones during the time of their consultancy with the Company. These milestones are during the first 12 months after the date of the agreement:
| · | Revenue Incentives “A” as defined above, with the exception that the common share awards are revised to 75,000 shares instead of 100,000, 40,000 instead of 50,000, 150,000 instead of 200,000 and 80,000 instead of 100,000. |
Our business plan contemplates additional increases in the number of employees and other personnel over the next 12-month period to enhance operational and our in-house R&D capacity. When beneficial to do so we will continue to outsource contract employment or engagements as needed. Additional capacity may be required with product advancement or retail acceptance of our new products, we may need to retain additional personnel particularly in the fields of product manufacturing, development, sales and distribution. It is not possible to accurately project potential needs into the future based on circumstances that may or may not occur.
Research and Development
Lexaria incurred $555,730 (2018 $492,864) in research and development expenditures during the period ending August 31, 2019. Specific R&D programs are in ongoing development and will be tightly related to our financial ability to undertake each research phase for each molecule. Due to our expanding portfolio coverage, we are continuing to examine accelerated timetable options for testing, research and development.
The Company’s earlier plans to include in vitro absorption tests of our patented technology of molecules such as: Vitamin E, Ibuprofen, and Nicotine allowed us to perform testing on Nicotine with positive results. Our plan to conduct our first ever in vivo absorption tests on CBD also yielded positive results. Ongoing testing plans are proceeding to further define molecular compatibility, absorption rates, timing and viable formats of delivery.
The Company continually focuses on new R&D programs to investigate potential additional commercial applications for its technology. These include, but are not limited to, ongoing programs to explore methods to integrate nanoemulsification chemistry techniques together with its technology that have demonstrated positive results to date, programs to further enhance intestinal bio absorption rates with its technology, as well as ongoing programs to expand the types and breadth of product form factors into which its technology can be applied. Depending on how many of these tests are undertaken, R&D budgets are expected to vary significantly. It is in our best interests to remain flexible at this early stage of our R&D efforts in order to capitalize on potential novel findings from early-stage tests and thus re-direct research into specific avenues that offer the most reward.
Subsidiaries
Lexaria has its wholly-owned subsidiaries; Lexaria CanPharm ULC, PoViva Corp., Lexaria Hemp Corp., Kelowna Management Services Corp. and Lexaria Pharmaceutical Corp, and our majority owned subsidiary Lexaria Nicotine LLC. On January 15, 2019, the Company announced the initial investment of $1,000,000 from Altria Ventures Inc., an indirect wholly-owned subsidiary of Altria Group, Inc., for a 16.667% equity interest along with certain other rights in Lexaria Nicotine LLC.
Much of the information included in this report includes or is based upon estimates, projections or other “forward looking statements”. Such forward looking statements include any projections or estimates made by us and our management in connection with our business operations. While these forward-looking statements, and any assumptions upon which they are based, are made in good faith and reflect our current judgment regarding the direction of our business, actual results will almost always vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested herein.

| Page 24 of 83 |
| Table of Contents |
Risks Associated with Our Business
Much of the information included in this quarterly report includes or is based upon estimates, projections or other “forward looking statements”. Such forward looking statements include any projections or estimates made by us and our management in connection with our business operations. While these forward-looking statements, and any assumptions upon which they are based, are made in good faith and reflect our current judgment regarding the direction of our business, actual results will almost always vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested herein.
Risks Associated with Our Business
Because there is no assurance that we will generate material revenues, we face a high risk of business failure.
There can be no assurance that our current or future products will be successful, and we cannot be sure that our overall business model within any particular sector will ever come to fruition, and if they do, will not decline over time. We may not recover all or any portion of our capital investment in product development, marketing, or other aspects of the business. Although we will exercise due consideration in our development of new products, and the marketing of them, ultimate consumer acceptance of these products is not reliably forecastable.
In addition, our product development plans may be curtailed, delayed or cancelled as a result of lack of adequate capital and other factors, such as weather, compliance with governmental regulations, current and forecasted prices for input costs of food products and changes in the estimates of costs to complete the projects. We will continue to gather information about our planned products, and it is possible that additional information may cause our company to alter our schedule or determine that a product should not be pursued at all. You should understand that our plans regarding our products are subject to change.
Our revenues now are primarily generated from out licensing of our technology. We should be considered to be a start-up: the revenue recognized for the period ended August 31, 2019 was $222,610.
Even if we develop food, consumer packaged goods (“CPG”) or intellectual property-based products or revenue streams, the potential profitability of each depends upon factors beyond the control of the Company.
The potential profitability of food and CPG products and of intellectual property revenue streams is dependent upon many factors beyond our control. For instance, prices and markets for food products are unpredictable, highly volatile, potentially subject to controls or any combination or other factors, and respond to changes in domestic, international, political, social and economic environments. These changes and events may materially affect our future financial performance. These factors cannot be accurately predicted and the combination of these factors may result in our company not receiving an adequate return on invested capital.
In addition, a product or technology that is initially successful and possibly even profitable may not remain so due to changes in consumer demand, regulatory environments, or other causes. There is no assurance that an initially successful product or technology will remain so.
Food, CPG and cannabis products are subject to comprehensive regulation which may cause substantial delays or require capital outlays in excess of those anticipated causing an adverse effect on our company.
Food, CPG and cannabis production, marketing, sales and safety operations, are subject to federal, state, and local laws relating to the protection of human health and safety. Food production and cannabis operations are each also subject to federal, state, and local laws and regulations which seek to maintain health and safety standards through a wide variety of regulations. Various permits from government bodies may be required by us in order to conduct our business. Regulations and standards imposed by federal, provincial, or local authorities may be changed at any moment in time and any such changes may have material adverse effects on our activities. Changes in regulations are impossible to foresee and could be disruptive or destructive to our business plans and execution. Moreover, compliance with such laws may cause substantial delays or require capital outlays in excess of those anticipated, thus causing an adverse effect on us. Additionally, we may be subject to liability for contaminants or other damages. To date, we have not been required to spend any material amount on compliance with environmental regulations. However, we may be required to do so in the future and this may affect our ability to expand or maintain our operations.

| Page 25 of 83 |
| Table of Contents |
We may not acquire market share or achieve profits due to competition in our industries.
Our company operates in highly competitive marketplaces with various competitors. Increased competition may result in reduced gross margins and/or loss of market share, either of which would seriously harm its business and results of operations. Management cannot be certain that the company will be able to compete against current or future competitors or that competitive pressure will not seriously harm its business. Some of our company’s competitors are much larger and have greater access to capital, sales, marketing and other resources. These competitors may be able to respond more rapidly to new regulations or devote greater resources to the development and promotion of their business model than the company can. Furthermore, some of these competitors may make acquisitions or establish co-operative relationships among themselves or with third-parties in the industry to increase their ability to rapidly gain market share.
Uncertain demand for our products or technology may cause our business plan to be unprofitable.
Demand for food products, CPG, technology delivery benefits and medical marijuana and cannabis or hemp related products is dependent on a number of social, political and economic factors that are beyond the control of our company. While we believe that demand for these products will continue to grow across North America, there is no assurance that such increase in demand will happen or that our endeavors will be profitable.
Without additional financing to develop our business plan, our business may fail.
Because we have generated only minimal revenue from our business and cannot anticipate when we will be able to generate meaningful revenue from our business, we will need to raise additional funds to conduct and grow our business. We do not currently have sufficient financial resources to completely fund the development of our business plan. We anticipate that we will need to raise further financing. We do not currently have any arrangements for financing and we can provide no assurance to investors that we will be able to find such financing if required. The most likely source of future funds presently available to us is through the sale of equity capital. Any sale of share capital will result in dilution to existing security-holders.
Our failure to protect our intellectual property may have a material adverse effect on our ability to develop and commercialize our products
Because patents involve complex legal and factual questions, the issuance, scope, validity, and enforceability of patents cannot be predicted with certainty.
Some of our patent pending applications may not be granted as patents. Even if patents are issued, they may not be issued with claims of sufficient breadth to protect our nutrient infusion technology or may not provide us with competitive advantage against competitors with similar products or technologies. Issued patents may be challenged, invalidated, or circumvented. If patents issued to us are invalidated or found to be unenforceable, we could lose the ability to exclude others from making, using or selling the inventions claimed. Moreover, an issued patent does not give us the right to use the patented technology or commercialize a product using the technology. Third-parties may have blocking patents that could be used to prevent us from developing our products, selling our products, or commercializing our nutrient infusion technology. Others may also independently develop products or technologies similar to those that we have developed or may reverse engineer or discover our trade secrets through proper means.
Enforcing a claim that a third-party infringes on, has illegally obtained or is using an intellectual property right, is expensive and time-consuming and the outcome is unpredictable. In addition, enforcing such a claim could divert management’s attention from our business. If any intellectual property rights were to be infringed, disclosed to, or independently developed by a competitor, our competitive position could be harmed. Any adverse outcome of such litigation or settlement of such dispute could subject us to significant liabilities and could put one or more of our patent pending applications at risk of being invalidated.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is risk that some of our confidential information could be compromised. This disclosure could provide our competitors with access to our proprietary information and may harm our competitive position.

| Page 26 of 83 |
| Table of Contents |
The failure to secure customers may cause our operations to fail.
We currently do not have many long-term agreements with any customers. Many of our products and services may be provided on a “onetime” basis. Accordingly, we will require new customers on a continuous basis to sustain our operations.
Because cannabis is a controlled substance in some regulatory jurisdictions our Third-Party Licensee’s operations may be subject to regulatory actions.
Lexaria and its subsidiaries are not involved directly or indirectly in the cultivation, processing, distribution, or utilization of cannabis or cannabis derived components. All of Lexaria’s consumer products utilize legally sourced hemp and hemp components in their production. Lexaria has an ancillary involvement exposure via out-licensing of its patented technology to licensees that may utilize the technology in the production of products that contain contents which are locally or state approved but federally controlled. Where licensee’s products contain controlled contents any revenue streams from such licensee’s may be interrupted by regulatory involvement in their business.
Lexaria has no knowledge of any non-compliance by any of its licensees with the regulatory framework(s) in which its licensee(s) operate.
There can be no assurance that we will develop any product that will meet with widespread consumer acceptance.
Both new and established food and CPG products fail to generate consumer interest on a regular basis. There is no assurance that a food or CPG product that is successfully adopted by consumers at one time; will still be in demand at a future time. If we cannot develop and sell products in commercial quantities, our business could fail.
The food CPG industries are highly competitive and there is no assurance that we will be successful in developing or successfully selling products.
The food and CPG industries are intensely competitive. We compete with numerous individuals and companies, including many food manufacturing and production companies, which have substantially greater technical, financial and operational resources and staff. Accordingly, there is a high degree of competition for desirable distribution channels, “shelf space” and salespeople in both the food and CPG industries. We cannot predict if the necessary funds can be raised to assist in our development of any distribution channels that may be helpful to our ability to generate sales and potential profits.
The marketability of food and CPG products will be affected by numerous factors beyond our control which may result in us not receiving an adequate return on invested capital to be profitable or viable.
The marketability of food and CPG products will be affected by numerous factors beyond our control. These factors include market fluctuations in consumer preferences for various food items based on factors such as pricing, macro trends for certain ingredients or flavors, ruling by regulators on health issues associated with certain foods, and more. The exact effect of these factors cannot be accurately predicted, but the combination of these factors may result in us not receiving an adequate return on invested capital to be profitable or viable.
If we are unable to hire and retain key personnel, we may not be able to implement our business plan.
Our success is largely dependent on our ability to hire highly qualified personnel. This is particularly true in those parts of our business that are related to intellectual property generation or exploitation. These individuals are in high demand and we may not be able to attract the personnel we need. In addition, we may not be able to afford the high salaries and fees demanded by qualified personnel, or may lose such employees after they are hired. Failure to hire key personnel when needed, or on acceptable terms, would have a significant negative effect on our business.
We are not the “operator” of vertically integrated food production facilities, and so we are exposed to the risks of our third-party operators.
We rely on the expertise of contracted third-parties for their judgment, experience and advice related to the manufacturing and/or packaging of our food products. We can give no assurance that these third-party operators or consultants will always act in our best interests, and we are exposed as a third-party to their operations and actions and advice in those operations and activities in which we are contractually bound.

| Page 27 of 83 |
| Table of Contents |
Our management has limited experience and training in the food processing and manufacturing industries, and in the cannabis products industries, and could make uninformed decisions that negatively impact our operations and our company.
Because our management has limited experience and training in the food processing and manufacturing industry, and in the cannabis products industry, we may not have sufficient expertise to make informed best practices decisions regarding our operations. It is possible that, due to our limited knowledge, we might elect to undergo manufacturing processes and incur financial burdens that a more experienced food manufacturing team might elect not to complete. Our ability to internally evaluate food and cannabis operations and opportunities could be less thorough than that of a more highly trained management team.
The possession, cultivation and distribution of marijuana may under certain circumstances lead to prosecution under United States federal law, which may cause our business to fail.
Over 30 US States, including our state of incorporation, Nevada, have approved and regulate medical marijuana use. Similarly, eleven states have approved and regulate non-medical marijuana use by adults. However, it remains illegal under United States federal law to grow, cultivate or sell marijuana for any purpose. In that regard, the United States Justice Department has released the COLE Memorandum of 8-29-13 which states that the Justice Department will not prioritize the prosecution of marijuana related activities authorized under state laws provided that state authorities implement and enforce strict guidelines to ensure the health, safety and security of the public. Where the individual state framework fails to protect the public, the Justice Department has instructed federal prosecutors to enforce the Controlled Substances Act of 1970. The Department of Justice has not, to our knowledge, published any policy or guidance specifically regarding the participation of a United States corporation in lawful medical marijuana related activities outside of the United States.
We do not currently, nor at any time in our corporate history have we ever cultivated, grown, processed, manufactured or sold marijuana in any location. Although we believe this fact to provide protection against prosecution related to marijuana legislation, we cannot provide any assurance to that effect. We do not hold a license in any jurisdiction enabling us to grow or sell marijuana or cannabis related edibles, but because of our business model we do not feel that is a barrier to entry for us. Instead, we plan to license our technology related to bio absorption of THC, to those entities that do have valid licenses in various North American jurisdictions to sell cannabis related edibles. If we are unable to license our technology to any valid license holders, then we may be shut out of this market.
Our company has no operating history and an evolving business model, which raises doubt about our ability to achieve profitability or obtain financing.
Our company has no significant history of operations in the legal medical marijuana sector, the legal hemp oil infused products sector, or in the food products sector. Moreover, our business model is still evolving and subject to change. Our company’s ability to continue as a going concern is dependent upon our ability to obtain adequate financing and/or to reach profitable levels of operations. In that regard we have no proven history of performance, earnings or success. There can be no assurance that we will achieve profitability or obtain future financing.
Conflicts of interest between our company and our directors and officers may result in a loss of business opportunity.
Our directors and officers are not obligated to commit their full time and attention to our business and, accordingly, they may encounter a conflict of interest in allocating their time between our future operations and those of other businesses. In the course of their other business activities, they may become aware of investment and business opportunities which may be appropriate for presentation to us as well as other entities to which they owe a fiduciary duty. As a result, they may have conflicts of interest in determining to which entity a particular business opportunity should be presented. They may also in the future become affiliated with entities, engaged in business activities similar to those we intend to conduct.
In general, officers and directors of a corporation are required to present business opportunities to a corporation if:
| · | The corporation could financially undertake the opportunity; |
|
|
|
| · | The opportunity is within the corporation’s line of business; and |
|
|
|
| · | It would be unfair to the corporation and its stockholders not to bring the opportunity to the attention of the corporation. |

| Page 28 of 83 |
| Table of Contents |
We have adopted a code of ethics that obligates our directors, officers and employees to disclose potential conflicts of interest and prohibits those persons from engaging in such transactions without our consent. Despite our intentions, conflicts of interest may nevertheless arise which may deprive our company of a business opportunity, which may impede the successful development of our business and negatively impact the value of an investment in our company.
Changing consumer preferences may cause our planned products to be unsuccessful in the marketplace.
The decision of a potential client to purchase our products may be motivated by cultural phenomena or by perceived health or nutritional benefits. The cultural desirability or popularity of hemp related products is subject to change due to factors beyond our immediate control. Similarly, the perceived nutritional or health related benefits of our products are subject to change in light of continuing research or the introduction of competitive products. Changes in consumer and commercial preferences, or trends, toward or away from cannabis or hemp related products would have a corresponding impact on the development of the market for our current and planned products. There can be no assurance that the products supplied by our company and or its partners will be successful in establishing or maintaining a significant share of the consumer market.
General economic factors may negatively impact the market for our planned products.
The willingness of businesses to spend time and money on non-essential food and health products may be dependent upon general economic conditions; and any material downturn may reduce the likelihood of consumers incurring costs toward what some may consider a discretionary expense item. Willingness by customers to buy our products may be dependent upon general economic conditions and any material downturn may reduce the potential profitability of the food sciences or medical marijuana business sectors.
A wide range of economic and logistical factors may negatively impact our operating results.
Our operating results will be affected by a wide variety of factors that could materially affect revenues and profitability, including the timing and cancellation of customer orders and projects, competitive pressures on pricing, availability of personnel, and market acceptance of our services. As a result, we may experience material fluctuations in future operating results on a quarterly and annual basis which could materially affect our business, financial condition and operating results.
Loss of consumer confidence in our company or in our industry may harm our business.
Demand for our services may be adversely affected if consumers lose confidence in the quality of our services or the industry’s practices. Adverse publicity may discourage businesses from buying our services and could have a material adverse effect on our financial condition and results of operations.
Unethical business practices may compromise the growth and development of our business.
The production and sale of medical marijuana is an emerging industry in which business practices are not yet standardized and are subject to frequent scrutiny and evaluation by federal, state, provincial, and municipal authorities, academics, and media outlets, among others. Although we intend to develop our business in accordance with best ethical practices, we may suffer negative publicity if we, our partners, contractors, or customers are found to have engaged in any environmentally, insensitive practices or other business practices that are viewed as unethical.
We could be required to enter into fixed price contracts which will expose us to significant market risk.
Fixed price contracts require the service provider to perform all agreed services for a specified lump-sum amount. We anticipate a material percentage of our services will be performed on a fixed price basis. Fixed price contracts expose us to some significant risks, including under-estimation of costs, ambiguities in specifications, unforeseen costs or difficulties, and delays beyond our control. These risks could lead to losses on contracts which may be substantial, and which could adversely affect the results of our operations.

| Page 29 of 83 |
| Table of Contents |
If we fail to effectively and efficiently advertise, the growth of our business may be compromised.
The future growth and profitability of our food and CPG products business and our technology licensing business will be dependent in part on the effectiveness and efficiency of our advertising and promotional expenditures, including our ability to (i) create greater awareness of our services, (ii) determine the appropriate creative message and media mix for future advertising expenditures, and (iii) effectively manage advertising and promotional costs in order to maintain acceptable operating margins. There can be no assurance that we will experience benefits from advertising and promotional expenditures in the future. In addition, no assurance can be given that our planned advertising and promotional expenditures will result in increased revenues, will generate levels of service and name awareness or that we will be able to manage such advertising and promotional expenditures on a cost-effective basis.
Our success is dependent on our unproven ability to attract qualified personnel.
We will depend on our ability to attract, retain and motivate our management team, consultants and other employees. There is strong competition for qualified technical and management personnel in the food science sector, and it is expected that such competition will increase. Our planned growth will place increased demands on our existing resources and will likely require the addition of technical personnel and the development of additional expertise by existing personnel. There can be no assurance that our compensation packages will be sufficient to ensure the continued availability of qualified personnel who are necessary for the development of our business.
We may not be able to obtain all of the licenses necessary to operate our business, which would cause our business to fail.
Our operations may require licenses and permits from various governmental authorities to conduct our business activities. We believe that we will be able to obtain all necessary licenses and permits under applicable laws and regulations for our operations and believe we will be able to comply in all material respects with the terms of such licenses and permits. However, such licenses and permits are subject to change in various circumstances. There can be no guarantee that we will be able to obtain or maintain all necessary licenses and permits.
If we fail to effectively manage our growth our future business results could be harmed and our managerial and operational resources may be strained.
As we proceed with our business plan, we expect to experience significant and rapid growth in the scope and complexity of our business. We will need to add staff to market our services, manage operations, handle sales and marketing efforts and perform finance and accounting functions. We will be required to hire a broad range of additional personnel in order to successfully advance our operations. This growth is likely to place a strain on our management and operational resources. The failure to develop and implement effective systems, or to hire and retain sufficient personnel for the performance of all of the functions necessary to effectively service and manage our potential business, or the failure to manage growth effectively, could have a materially adverse effect on our business and financial condition.
Risks Associated with Our Common Stock
Trading on the OTCQX and CSE may be volatile and sporadic, which could depress the market price of our common stock and make it difficult for our stockholders to resell their shares.
Our common stock is quoted on the OTCQX electronic quotation service operated by OTC Markets Group Inc. Trading in stock quoted on the OTCQX is often thin and characterized by wide fluctuations in trading prices, due to many factors that may have little to do with our operations or business prospects. This volatility could depress the market price of our common stock for reasons unrelated to operating performance. Moreover, the OTCQX is not a stock exchange, and trading of securities on the OTCQX is often more sporadic than the trading of securities listed on a quotation system like NASDAQ or a stock exchange like Amex. Accordingly, shareholders may have difficulty reselling any of the shares.

| Page 30 of 83 |
| Table of Contents |
Our stock is a penny stock. Trading of our stock may be restricted by the Securities and Exchange Commission’s penny stock regulations which may limit a stockholder’s ability to buy and sell our stock.
Our stock is a penny stock. The Securities and Exchange Commission has adopted Rule 15g-9 which generally defines “penny stock” to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. Our securities are covered by the penny stock rules, which impose additional sales practice requirements on broker-dealers who sell to persons other than established customers and “accredited investors”. The term “accredited investor” refers generally to institutions with assets in excess of $5,000,000 or individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouse. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a form prepared by the Securities and Exchange Commission which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer’s confirmation. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the stock that is subject to these penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules discourage investor interest in and limit the marketability of our common stock.
The Financial Industry Regulatory Authority, or FINRA, has adopted sales practice requirements which may also limit a stockholder’s ability to buy and sell our stock.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
The speculative nature of our business plan may result in the loss of your investment.
Our operations are in the start-up stage only and are unproven. We may not be successful in implementing our business plan to become profitable. There may be less demand for our services than we anticipate. There is no assurance that our business will succeed and you may lose your entire investment.
Because we do not intend to pay any dividends on our shares, investors seeking dividend income or liquidity should not purchase our shares.
We have not declared or paid any dividends on our shares since inception, and do not anticipate paying any such dividends for the foreseeable future. We presently do not anticipate that we will pay dividends on any of our common stock in the foreseeable future. If payment of dividends does occur at some point in the future, it would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any common stock dividends will be within the discretion of our Board of Directors. We presently intend to retain all earnings to implement our business plan; accordingly, we do not anticipate the declaration of any dividends for common stock in the foreseeable future.
Investors seeking dividend income or liquidity should not invest in our shares.
Because we can issue additional shares, purchasers of our shares may incur immediate dilution and may experience further dilution.
We are authorized to issue up to 220,000,000 shares. The board of directors of our company has the authority to approve additional share issuances, and to determine the rights, preferences and privileges of such shares, without consent of any of our stockholders. Consequently, our stockholders may experience more dilution in their ownership of our company in the future.

| Page 31 of 83 |
| Table of Contents |
Other Risks
Protection against environmental risks.
We believe that our operations comply, in all material respects, with all applicable environmental regulations.
Our operating partners maintain insurance coverage customary to the industry; however, we are not fully insured against all possible environmental risks.
Any change to government regulation/administrative practices may have a negative impact on our ability to operate and our profitability.
The laws, regulations, policies or current administrative practices of any government body, organization or regulatory agency in the United States, Canada, or any other jurisdiction, may be changed, applied or interpreted in a manner which will fundamentally alter the ability of our company to carry on our business.
The actions, policies or regulations, or changes thereto, of any government body or regulatory agency, or other special interest groups, may have a detrimental effect on us. Any or all of these situations may have a negative impact on our ability to operate and/or our profitably.
Investors’ interests in our company will be diluted and investors may suffer dilution in their net book value per share if we issue additional shares or raise funds through the sale of equity securities.
Our constating documents authorize the issuance of 220,000,000 shares of common stock with a par value of $0.001. In the event that we are required to issue any additional shares or enter into private placements to raise financing through the sale of equity securities, investors’ interests in our company will be diluted and investors may suffer dilution in their net book value per share depending on the price at which such securities are sold. If we issue any such additional shares, such issuances also will cause a reduction in the proportionate ownership and voting power of all other shareholders. Further, any such issuance may result in a change in our control.
Our by-laws do not contain anti-takeover provisions, which could result in a change of our management and directors if there is a take-over of our company.
We do not currently have a shareholder rights plan or any anti-takeover provisions in our By-laws. Without any anti-takeover provisions, there is no deterrent for a take-over of our company, which may result in a change in our management and directors.
The majority of our directors and officers are residents of other countries other than the United States, as a result, investors may find it difficult to enforce, within the United States, any judgments obtained against our company or our directors and officers.
Our head office and the majority of our assets are located in Kelowna, British Columbia and we lease administrative office space in Phoenix, Arizona. In addition, a majority of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons’ assets are located outside the United States. As a result, it may be difficult for investors to enforce within the United States any judgments obtained against our company or our officers or directors, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof.
Our by-laws contain provisions indemnifying our officers and directors against all costs, charges and expenses incurred by them.
Our by-laws contain provisions with respect to the indemnification of our officers and directors against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, actually and reasonably incurred by him, including an amount paid to settle an action or satisfy a judgment in a civil, criminal or administrative action or proceeding to which he is made a party by reason of his being or having been one of our directors or officers.
Trends, risks and uncertainties.
We have sought to identify what we believe to be the most significant risks to our business, but we cannot predict whether, or to what extent, any of such risks may be realized nor can we guarantee that we have identified all possible risks that might arise. Investors should carefully consider all of such risk factors before making an investment decision with respect to our common shares.

| Page 32 of 83 |
| Table of Contents |
Item 1B. Unresolved Staff Comments
None.
Executive Offices
The address of our principal executive office is Unit 100–740 McCurdy Road, Kelowna BC V1X 2P7 with rent of CAD$4,823. We have administrative functions located in Phoenix, Arizona at nominal cost. Our telephone number is (250) 765 6424.
Significant Acquisitions and Dispositions
We have leased a new head-office location in Kelowna, Canada, that included the purchase and construction of office equipment, furniture, computers, and communications systems. We also constructed space for a Canadian federal licensed laboratory on-premises for our internal R&D purposes, for which a license has been received from Health Canada. Costs incurred to date are included in Capitalized Assets in the financial statements and notes.
We know of no other material, existing or pending legal proceedings against our company, nor are we involved as a plaintiff in any other material proceeding or pending litigation. There are no other proceedings in which any of our directors, executive officers or affiliates, or any registered or beneficial stockholder, is an adverse party or has a material interest adverse to our interest.
Item 4. Mine Safety Disclosures
Not Applicable.

| Page 33 of 83 |
| Table of Contents |
PART II
Our common shares are quoted on the OTCQX under the symbol “LXRP.” Our common shares are also quoted on the Canadian Securities Exchange under the symbol “LXX”. The following quotations, obtained from Yahoo Finance, reflect the high and low bids for our common shares as quoted on the OTCQX based on inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
The high and low bid prices of our common stock for the periods indicated below are as follows:
OTC Bulletin Board (1) | ||
Quarter Ended | High | Low |
November 30, 2016 | $0.35 | $0.11 |
February 28, 2017 | $0.699 | $0.20 |
May 31, 2017 | $0.625 | $0.27 |
August 31, 2017 | $0.43 | $0.27 |
November 30, 2017 | $1.01 | $0.32 |
February 28, 2018 | $2.54 | $0.82 |
May 31, 2018 | $1.65 | $0.78 |
August 31, 2018 | $2.43 | $1.50 |
November 30, 2018 | $2.20 | $0.98 |
February 28, 2019 | $1.70 | $0.75 |
May 31, 2019 | $1.34 | $0.81 |
August 31, 2019 | $1.00 | $0.60 |
(1) Over-the-counter market quotations reflect inter-dealer prices without retail mark-up, mark-down or commission, and may not represent actual transactions | ||
As of November 5, 2019, there were 56 holders of record of our common stock. As of such date, 78,787,134 shares of common stock were issued and outstanding.
Dividend Policy
We have not paid any cash dividends on our common stock and have no present intention of paying any dividends on the shares of our common stock. Our current policy is to retain earnings, if any, for use in our operations and in the development of our business. Our future dividend policy will be determined from time to time by our board of directors.
Recent Sales of Unregistered Securities
Other than set out below, we did not sell any equity securities which were not registered under the Securities Act during the year ended August 31, 2019 that were not otherwise disclosed on our quarterly reports on Form 10-Q or our current reports on Form 8-K filed during the year ended August 31, 2019.

| Page 34 of 83 |
| Table of Contents |
A summary of the activity is set out in the table below:
Type of Issuance |
| Number of Shares |
|
| Total Value |
| ||
Warrant exercise(1) |
|
| 1,626,513 |
|
|
| 796,122 |
|
Option exercise |
|
| 430,000 |
|
|
| 66,250 |
|
Private placement |
|
| 947,150 |
|
|
| 1,515,440 |
|
Per agreements(2) |
|
| 250,000 |
|
|
| 234,500 |
|
|
|
| 3,253,663 |
|
|
| 2,612,312 |
|
| (1) Includes 384,212 broker warrants exercised for gross proceeds of $191,742 | |
| (2) The Company awarded the restricted common shares as required by consulting contracts. |
Warrants
Lexaria awarded 100,000 warrants with an exercise price of $0.96 and an expiration date of May 21, 2021 to a vendor, pursuant to an investor relations contract. The warrants were valued at $52,817 and included in investor relations.
During the period ended August 31, 2019 the Company issued 28,175 warrants with an exercise price of $1.21 expiring October 3, 2019, 107,737 warrants at $0.60 expiring April 3, 2019. These warrants were valued at $22,579 and recorded as a share issue cost within additional paid in capital for a net effect of $Nil.
Equity Compensation Plan Information
We have no long-term incentive plans other than the stock option plans described below updated for issuable options as at August 31, 2019:
2007 Equity Plan
On April 25, 2007, our shareholders approved our 2007 Equity Incentive Stock Option Plan.
The 2007 Plan permits our company to issue up to the remaining 412,500 shares of our common stock to eligible employees and directors of our company.
2010 Equity Compensation Plan
On February 26, 2010, our shareholders approved and adopted our 2010 equity incentive plan.
The 2010 Plan permits our Company to issue up to the remaining 1,512,500 shares of our common stock to directors, officers, employees and eligible consultants of our Company upon exercise of stock options granted under the 2010 plan.
2014 Stock Option Plan
On June 11, 2014, our shareholders approved and adopted our 2014 Stock Option Plan which permits our company to grant up to an aggregate of the remaining 2,107,500 options to acquire shares of our common stock, to directors, officers, employees and consultants of our company.
Equity Incentive Plan
On June 20, 2019 our shareholders approved and adopted our Equity Incentive Plan whereby the board of directors may, from time to time, grant up to the remaining 7,838,713 stock options to directors, officers, employees, and consultants.
The Board may amend, subject to the approval of any regulatory authority whose approval is required, suspend or terminate this Plan or any portion thereof. No such amendment, suspension or termination shall alter or impair any outstanding unexercised Options or any rights without the consent of such Participant. If this Plan is suspended or terminated, the provisions of this Plan and any administrative guidelines, rules and regulations relating to this Plan shall continue in effect for the duration of such time as any Option remains outstanding.

| Page 35 of 83 |
| Table of Contents |
It is the Company’s intent to terminate the 2007 Plan, the 2010 Plan and the 2014 Plan upon the expiration of all options currently issued and outstanding under such plans. All future option issuances shall be made under the Equity Incentive Plan.
Securities authorized for issuance under equity compensation plans | |||
Plan Category | Number of securities to be based upon exercise of outstanding options, warrants and rights | Weighed-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plan [excluding securities reflected in column (a)] |
Equity compensation plans not approved by shareholders | Nil | Nil | Nil |
Equity compensation plans approved by shareholders |
| ||
2007 Equity compensation plan | 300,000 | $1.53 | 112,500 |
2010 Equity compensation plan | 1,325,000 | $1.53 | 187,500 |
2014 Stock Option Plan approved by security holders | 1,990,000 | $0.36 | 117,500 |
Equity Incentive Plan | 1,388,000 | $0.92 | 6,450,713 |
Total | 5,003,000 | $0.89 | 6,868,213 |
Convertible Securities
As of August 31, 2019, we had outstanding options to purchase 5,003,000 shares of our common stock exercisable between prices of $0.10 and $1.53.
During the year ended August 31, 2019, the Company pursuant to existing stock option plans, granted stock options to directors, officers, employees and consultants that enable the option holders to purchase up to 350,000 common shares of the Company at a price of $0.99 and 100,000 common shares at $0.81 for a period of five years, vesting immediately. Vesting stock options were also granted to purchase: 440,000 common shares at $0.99, 390,000 at $1.27 (which were subsequently cancelled and re-issued at $0.99), 240,000 at $1.06, 30,000 at $1.16, 48,000 at $0.96 and 450,000 at $0.81 that vest over three years. The vested options were valued at $368,115 and included in consulting expense and R&D. Vesting options granted in a prior period were valued at $22,197 and included in consulting expense.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
We did not purchase any of our shares of common stock or other securities during our fiscal year ended August 31, 2019.
Item 6. Selected Financial Data
Not applicable. The Company qualifies as a “Smaller Reporting Company” and, accordingly, this Item and the related disclosure is not required.

| Page 36 of 83 |
| Table of Contents |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read in conjunction with our audited consolidated financial statements and the related notes that appear elsewhere in this annual report. The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to such differences include but are not limited to; those discussed below and elsewhere in this annual report, particularly in the section entitled “Risk Factors”.
Our audited financial statements are stated in United States Dollars and are prepared in accordance with United States Generally Accepted Accounting Principles.
Plan of Operation
During the next twelve-month period (beginning September 1, 2019), we intend to:
· continue sales and marketing efforts for consumer product lines · pursue technology out-licensing opportunities for our patented DehydraTECH technology. This will be focused first primarily on the cannabinoid and nicotine sectors, and will evolve as time allows for completed R&D in other sectors, to the NSAID, and vitamin sectors; · identify and secure sources of equity and/or debt financing for intellectual property pursuit and maintenance, R&D, and consumer product formulation and marketing;
Our plans beyond fiscal 2021 are dependent upon our ability to obtain sufficient capital through equity capital or other and by revenues generation to execute. During the previous year we did raise sufficient capital to fulfill all our plans. Without sufficient capital, our plans will change, and could change materially. We anticipate that we will incur up to the following operating expenses during this period:
Estimated Funding Required During the 12 Months beginning September 1, 2019 | |||||||
Expense |
| Amount ($) |
|
| Estimated Completion/Due Date | ||
Research and Development of additional products Research and Development (General) |
| 70,000 800,000 |
|
| 12 months 12 months | ||
Patent applications and trademark |
|
| 300,000 |
|
| 12 months | |
Marketing and Sales |
|
| 200,000 |
|
| 12 months | |
Consulting Fees (-50% in officers and directors) |
|
| 1,200,000 |
|
| 12 months | |
Wages and Salaries |
|
| 575,000 |
|
| 12 months | |
Professional Fees |
|
| 160,000 |
|
| 12 months | |
Rent |
|
| 45,000 |
|
| 12 months | |
Other general administrative expenses (including travel, insurance, conferences, and fees) |
|
| 300,000 |
|
| 12 months | |
Interest Expense |
|
| 10,000 |
|
| 12 months | |
Total |
|
| 3,660,000 |
|
|
| |
12 Month Outlook for Current Product Line, Product Development & Design, Patents
As at August 31, 2019, we had a working capital surplus of $1,634,322 and cash on hand of $1,285,147. We therefore estimate that we will require approximately $2.5 million in cash to finance our planned expenditures for the 12 months beginning September 1, 2019. In the uncertain event that we are unable to raise sufficient funds to execute our current business plan, we will scale back our operations to prioritize immediate and necessary expenses, shifting portions of our plan into our longer term planning for fiscal 2021. We estimate our minimum necessary expenses for the year to be roughly $2.5 million. These necessary expenses include professional fees, wages and general and administrative expenses necessary to satisfy our public reporting requirements.

| Page 37 of 83 |
| Table of Contents |
Our business strategy involves several elements. We intend to prioritize our revenue generating efforts in 2020/21 on technology licensing in the nicotine, hemp and cannabinoid sectors, with a secondary focus on our consumer food products enriched with full spectrum hemp oil. Revenues from existing licensees are anticipated to increase based on ongoing usage fees as licensees begin or ramp up products or contracted minimum requirements become due.
Our patented technology was developed to aid absorption and bioavailability of certain “payload” molecules, including cannabinoids such as CBD and THC. CBD is found in plant species such as hemp, cannabis, and Echinacea, is not psychoactive and may have desirable qualities. Our technology appears to improve absorption and bioavailability of CBD into human epi-intestinal cells. We are developing a line of food products fortified with full spectrum hemp oil that contains cannabinoids such as CBD, but contains less than 0.3% THC. Because of the low amounts of THC, and because the hemp oil is derived from legal hemp, our research into the products is legal under US federal law.
We first began selling trial amounts of ViPova branded black tea fortified with hemp oil and utilizing our technology, in January 2015 and added additional flavours over time. We currently sell three flavors of ViPova tea. Sales of these products have been modest but are expected to improve in the long term.
We also began offering our first coffee and hot chocolate also fortified with full spectrum hemp oil, and also under the ViPova brand. Together, tea, coffee and hot chocolate comprise all our product offerings under the ViPova brand, despite modest changes to flavors or packaging, etc. Offering a variety of self-made beverages to the consumers helps us to establish the ViPova brand and may also help us to develop relationships with retail distributors who are less likely to place orders from manufacturers that can only offer a single product.
Generating meaningful revenue from product sales will be challenging and will rely in part on our ability to achieve widespread retail distribution access, which to date we have failed to achieve. We are also investigating the possibility of generating sales from international markets, in those locations where hemp oil fortified foods are permissible by law.
ViPova branded products are owned by our wholly owned Poviva Corp subsidiary.
While the ViPova line is focused on a “coffee house” experience, the Lexaria Energy line is focused on athletic performance and active lifestyle needs. The first Lexaria Energy product was believed to be unique or nearly so: a protein energy bar utilizing our technology to fortify it with full spectrum hemp oil. We first offered the Lexaria Energy Bar for sale in November 2015, but it was since discontinued due to the complexities in locating reliable manufacturing. TurboCBD and ChrgD+ products have also been publicly released demonstrating additional product formats that benefit from our patented DehydraTECH technology’s advantages in capsules and powdered form for ready to drink beverages respectively.
Lexaria Energy, TurboCBD and ChrgD+ branded products are owned 100% by Lexaria Bioscience Corp.
A manufacturing facility was contracted to produce the Lexaria Energy bar in 2015. Recipes have evolved and at the time of this report the Company had no inventories of protein bars to be offered for sales and was negotiating for a suitable manufacturing facility and prices.
Our strategy is to encourage online sales via dedicated websites, and also to pursue traditional grocery store, convenience store, specialty stores, roadside store and wholesale distribution channels. To facilitate distribution, we have third-party fulfillment centers that process and ship orders.
It is our intention, subject to sufficient funding being available, to provide R&D to develop additional products under our brands, such as protein powders for shakes or smoothies, protein energy drinks, mouth melts, and intermediary materials that clientele can purchase as a raw material to use in making their own products. We are also pursuing other product development and expect to launch new products.
Through our product development we have communicated to the industry the versatility of our technology in specific CPG formats and we believe this strategy has been successful in assisting us in technology licensing discussions with potential new clients. We believe the range of products available and under development are sufficient to prepare for revenue growth and potentially profitable long-term operations if we are able to generate sufficient consumer and business clientele demand.
Meanwhile, our business strategy contains a second element that we believe will be more impactful to future corporate growth that involves the further development and out-licensing of our intellectual property of molecule delivery that enhances bioactivity or absorption.

| Page 38 of 83 |
| Table of Contents |
At this time we are not planning to offer for sale any products containing THC in quantities higher than 0.3%. However, we are continuing to expand the number of licensees licensing our technology that are legally state-licensed to offer THC products in the states or international jurisdictions where they do business. We also plan to license our technology to other companies for the delivery of molecules other than THC or cannabinoids, such as nicotine that we have licensed to Altria Ventures Inc., an indirect wholly-owned subsidiary of Altria Group, Inc. Our October 31, 2017 announcement of the USPTO Notice of Allowance for our first patent granted and the subsequent 15 granted patents of our technology related to new molecule groups, along with our ongoing patent filing and grants, may enhance our ability to successfully pursue this initiative during fiscal 2020.
We continue to communicate the benefits of our technology to potential licensing partners, i.e. with higher absorption levels a manufacturer could perhaps infuse smaller amounts of active molecules into a product, thus reducing their manufacturing input costs, to provide higher bioavailability with the dosing limits being imposed or contemplated in many jurisdictions, to infuse beverages while masking the flavor and smell of the active molecules, and predictable delivery times. We believe these to be meaningful competitive advantages that may lead to the potential to generate licensing revenue, and will pursue these opportunities within the cannabinoids, nicotine and other bioactive molecular markets both within the USA and also internationally, in those locations where they are legal and regulated by government.
We will not sell any THC products – we only license technology to already-licensed participants in valid jurisdictions. We expect a low number of licensees initially and currently have nine revenue generating agreements with such licensees and additional letters of intent and negotiations with other potential licensees. We do not sell any nicotine products and do not intend to – however our joint venture partner may elect to utilize our technology in products containing nicotine for sale to consumers in the USA or internationally.
Subject to budgetary availability, we also plan to conduct additional in vitro and in vivo studies testing the absorption of some or all of the molecules named within our patent applications – CBD, NSAIDs, Vitamins, PDE5 inhibitors and Nicotine – to substantiate the effectiveness of our technology. More than simply satisfying scientific curiosity, successful tests could lead to increased awareness and acceptance of our technology as a meaningful method by which to deliver some or all of the named molecules more effectively than their current delivery methods. Therefore, absorption tests could become an important element leading towards higher rates of acceptance of our technology licensing initiatives.
We will pursue technology licensing opportunities as a method of generating highly profitable revenue streams over long periods of time. In addition, while eight of our US patents and eight of our Australian patents have been granted to date, we have multiple other applications filed in the US and around the world. It is not possible to forecast with certainty when, or if, our remaining patents pending will become granted patents. But if our remaining patent applications do become granted patents, our ability to generate meaningful license revenue from our intellectual property may increase in a very short period of time.
We will continue to pursue our remaining patents pending as vigorously as we are able, since the successful granting of more of those applications could lead to material increases in shareholder value. We are pursuing patent protection in more than 40 countries around the world.
Results of Operations for our Year Ended August 31, 2019 and August 31, 2018
Our net loss and comprehensive loss and the changes between those periods for the respective items are summarized as follows:
|
| YEAR ENDED |
|
| YEAR ENDED |
|
|
|
| |||
|
| August 31 |
|
| August 31 |
|
|
|
| |||
|
| 2019 |
|
| 2018 |
|
| Change |
| |||
Revenue |
| $ | 222,610 |
|
| $ | 433,287 |
|
| $ | (210,677 | ) |
General and administrative |
|
| 4,358,130 |
|
|
| 7,017,289 |
|
|
| (2,659,159 | ) |
Consulting fees & Employees |
|
| 1,777,934 |
|
|
| 5,332,398 |
|
|
| (3,554,464 | ) |
Legal and professional |
|
| 670,863 |
|
|
| 289,062 |
|
|
| 381,801 |
|
Net Loss |
| $ | (4,158,413 | ) |
| $ | (6,609,186 | ) |
| $ | 2,450,773 |
|
Revenue
Licensing revenues of $198,000 represent the majority of revenues during the year ended August 31, 2019 and reflect delays in usage fee revenues from existing licensees in Canada waiting for approval from Health Canada on products, and other licensees initiating or ramping up their production. Revenue was primarily based on new licence agreements entered into recognising the IP Territory Licensing fee, and existing licenses generating usage fees. Increasing ongoing usage fees are expected as licensees begin or ramp up products or contracted minimum requirements become due.

| Page 39 of 83 |
| Table of Contents |
Two years ago the Company had one Licensee and today we have nine Licensees. The territory fees consist of IP licensing fees for the transfer of the Technology at the signing of definitive agreements for the DehydraTECH technology. The additional Licensing fees include payments due upon transfer of the technology and installment payments that are receivable within 12 months (Note 7). We are pleased that we have signed additional licenses and are looking toward revenues increasing during fiscal 2020 with the legalization of edible products in Canada expected during October 2019 and the potential for licensee product launches early in calendar 2020 in that country. Our additional and expanded licenses in the US are anticipated to generate ongoing usage fee revenues based on contracted minimums or based on licensee sales starting during our fiscal 2020.
We have made progress in signing more corporate licensees than ever before in our corporate history, but most of these licensees are small start up companies that continue to present operational risk to us. We continue to attempt to work with larger more established companies to encourage them to adopt our technology, but the markets have been slow to adopt our technology, notwithstanding our new corporate relationship with a Fortune 500 company in the nicotine industry.
Consumer product sales remain low due to ongoing challenges in securing expansive distribution opportunities, third-party production challenges, inconsistent federal vs. state or local regulations, and payment processing changes. The Company continues to pursue more widespread distribution possibilities which have the potential to unlock more significant consumer product revenues.
During the year ended August 31, 2019, our revenues were derived within the following categories: $198,000 (2018 $415,183) of intellectual property licensing revenue and $24,610 (2018 $18,104) in product and other revenues.
As fiscal 2019 came to a close, hemp oil fortified foods, and hemp seed products continued gaining consumer acceptance and provide a reason to believe that sales could increase. In addition, legislative trends in America and in many nations around the world such as Canada and the UK are supportive of additional opportunities in the hemp-based foods and supplements sector. Those trends could support higher potential consumer product sales. Release of the ChrgD+ product was successful, but sales were limited due to ongoing payment processing issues outside of the Company’s control, and due to our not being successful in obtaining widespread retail distribution channels.
For 2020 the Company expects to continue to derive the majority of its revenues from technology licensing to third parties noting that IP territory fees are recognized when new definitive license agreements occur and IP usage fees are dependent upon our licensees’ opportunity to implement the technology pursuant to applicable regulatory approvals. Canadian regulatory approval for ingestible products was originally scheduled for October 17, 2019, but there are indications that actual individual product approvals required from Health Canada may delay licensee product launches into 2020 in that country. At August 31, 2015 the Company had zero technology licensing agreements entered. By August 31, 2016 we had entered several LOI’s or definitive agreements related to technology out-licensing. During the period ended August 31, 2019 we entered into nine active licensing agreements that are expected to generate additional revenue from the payment of usage fees as the licensees’ production and sales occur. It is the Company’s view its eight US patents granted and eight Australian patents granted along with its expanding patent portfolio is a positive step in enabling the generation of more significant revenues during fiscal 2020. At the time of this report the Company has entered more than 10 formal letters of intent or definitive agreements and is negotiating more.
We do not expect that all of the letters of intent into which we enter will result in definitive agreements with paying customers and cannot predict how many will. We believe that strengthening and expanding our intellectual property portfolio and conducting supportive R&D will jointly contribute to strengthening revenue prospects.
General and Administrative
Our general and administrative expenses decreased by $2,659,158 during the year ended August 31, 2019. The decrease in our general and administrative expenses was largely due to non-cash expenses related to valuation of grants for service and share-based payments required by contracts included in fiscal 2018. Increases during fiscal 2019 included expanded patent applications, R&D, IR programs and the addition of employees for a total of $1,061,125, which includes $368,115 of non-cash compensation and $58,243 increase in depreciation related to new facilities and equipment.
Interest Expense
Interest expense for the year ended August 31, 2019 was $Nil (2018 $Nil). The Company has no debt at this time other than month-to-month receivables.

| Page 40 of 83 |
| Table of Contents |
Consulting fees
Our consulting fees decreased by $3,887,663 primarily due to the non-cash payments for services included in fiscal 2018. Our executives are typically consultants and costs associated with those agreements comprise a significant portion of our consulting fees expense (Note 16).
Legal and Professional Fees
Our professional fees increased by $381,801 to $670,863 during the year primarily due to ongoing patent and trademark filings, consultations on licensing agreements, and other advisory services. Although we always try to minimize expenses, we consider increases in costs related to patent and trademark work to reflect positive progress in executing our business plan. We recognize certain legal fees, tax advice fees, and accounting services all as “Professional Fees.”
Liquidity and Financial Condition
|
| August 31 |
|
| August 31 |
| ||
Working Capital |
| 2019 |
|
| 2018 |
| ||
Current assets |
| $ | 1,818,829 |
|
| $ | 2,284,051 |
|
Current liabilities |
| $ | (184,507 | ) |
| $ | (43,640 | ) |
Net Working Capital |
| $ | 1,634,322 |
|
| $ | 2,240,411 |
|
The Company’s working capital balance decrease during the year was limited due to exercises of outstanding options and warrants and the private placement (Note 13) completed during the year. The Company maintained a positive and strong working capital position throughout the year.
|
| August 31 |
|
| August 31 |
| ||
Cash Flows |
| 2019 |
|
| 2018 |
| ||
Cash flows (used in) provided by operating activities |
| $ | (3,005,555 | ) |
| $ | (2,517,979 | ) |
Cash flows (used in) provided by investing activities |
| $ | (769,165 | ) |
| $ | (155,399 | ) |
Cash flows (used in) provided by financing activities |
| $ | 3,332,683 |
|
| $ | 1,867,224 |
|
Decrease in cash |
| $ | (442,037 | ) |
| $ | (806,153 | ) |
Operating Activities
Net cash used in operating activities was $3,005,555 for the year compared with cash used in operating activities of $2,517,979 during the same period in 2018. This difference was largely due to the increased costs pertaining to consulting, advertising and promotion, patent and trademark related filings, legal advisory services, new employees, research and development, and travel.
Investing Activities
Net cash used in investing activities was $769,165 (2018 $155,399) for the year due to the Company’s cost incurred related to its patent applications $122,982 and our new office space and equipment (Note 10) $646,183.
Financing Activities
Net cash provided from financing activities was $3,332,683 during the year ended August 31, 2019 compared to net cash provided of $1,867,224 during the same period in 2018.

| Page 41 of 83 |
| Table of Contents |
Results of Operations for our Year Ended August 31, 2018 and August 31, 2017
Our net loss and comprehensive loss for the year ended August 31, 2018, for the year ended August 31, 2017 and the changes between those periods for the respective items are summarized as follows:
|
| Year Ended August 31, 2018 $ |
|
| Year Ended August 31, 2017 $ |
|
| Change $ |
| |||
Revenue |
|
| 433,287 |
|
|
| 63,639 |
|
|
| 369,648 |
|
General and administrative |
|
| 7,017,289 |
|
|
| 1,963,354 |
|
|
| 5,053,935 |
|
Interest expense |
|
| - |
|
|
| 6,015 |
|
|
| (6,015 | ) |
Consulting fees |
|
| 5,332,398 |
|
|
| 1,017,872 |
|
|
| 4,314,526 |
|
Legal and Professional fees |
|
| 289,062 |
|
|
| 210,297 |
|
|
| 78,765 |
|
Net Loss |
|
| (6,609,186 | ) |
|
| (1,929,465 | ) |
|
| (4,679,721 | ) |
Revenue
Licensing revenues represent the majority of the $433,287 in revenues during the year ended August 31, 2018 and illustrate a significant gain from the previous year. Revenue increases were primarily based on new licence agreements entered into recognising the IP Territory Licensing fee and they are expected to generate future ongoing IP Usage Licensing fees.
One year ago the Company had one Licensee and today we have five Licensees. The Territory fees consist of IP licensing fees for the transfer of the Technology with the signing of definitive agreements for the DehydraTECH™ technology with: the Cannfections Group Inc. for a 7-year term for infused chocolates and candies to be developed and sold in Canada and internationally, NeutriSci International Inc. for a 2-year term for the manufacturing and sale of CBD based products, Biolog, Inc. for a 5-year term to manufacture food and beverage infused products to be sold in the United States, GP Holdings LLC for infused beverages and topical skin products for a 5-year term (subsequent to August 31, 2018 we cancelled this contract due to non-performance), Nuka Enterprises LLC for their 1906 Chocolates for 10 years renewing from their chocolate only 2 year contract to now include chocolate, candies, beverages, capsules and pills, and topical creams, and Hill Street Beverage Company for a 5-year term for infused alcohol-free beverages in Canada. The additional Licensing fees include payments due upon transfer of the Technology and installment payments that are receivable within 12 months (Note 7). We are pleased that our licensing revenues are increasing in scale and across a larger number of customers although they have not grown as quickly as anticipated.
Consumer product sales remain low due to challenges in securing expansive distribution opportunities, 3rd-party production challenges, inconsistent federal vs. state or local regulations, and payment processing changes. The Company continues to pursue more widespread distribution possibilities which have the potential to unlock more significant consumer product revenues.
During the year ended August 31, 2018, our revenues were derived within the following categories: $415,183 (2017 $45,809) (an 806% increase year over year) of licensing revenue and $18,104 (2017 $17,830) (a 1.5% increase year over year) in product and other revenues.
As fiscal 2018 came to a close, hemp oil fortified foods, and hemp seed products continued gaining consumer acceptance and provide a reason to believe that sales could increase. In addition, legislative trends in America and in many nations around the world such as Canada and the UK are supportive of additional opportunities in the hemp-based foods and supplements sector. Those trends could support higher potential consumer product sales. Release of the TurboCBDTM product was successful but sales were limited by changes to payment processing services outside of the Company’s control. At the time of this report the Company had extinguished its supplies of certain products like protein bars and the lack of inventory was also a negative impact on consumer product sales potential.
For 2019 the Company expects to continue to derive the majority of its revenues from technology licensing to third parties noting that IP Territory fees are recognized when new definitive license agreements occur and IP Usage fees are dependent up on licensees opportunity to implements the technology based upon regulatory approval. Canadian regulatory approval for ingestible products is anticipated within 12 months of the October 17, 2018 legalization of recreational cannabis in that country. At August 31, 2015 the Company had zero technology licensing agreements entered. By August 31, 2016 we had entered several LOI’s or definitive agreements related to technology out-licensing. During the period ended August 31, 2018 we have entered into six new licensing agreements that increased our IP licensing revenue and we expect additional revenue will be generated from the licensees utilizing the technology in their processes from the usage fees as their production and sales occur. It is the Company’s view that the December 9, 2017, grant of patent US 9839612 B2, and the grants of US 9972680 B2 and US 9974739 B2 during May 2018 and its expanding patent portfolio is a positive step in enabling the generation of more significant revenues during fiscal 2018. At the time of this report the Company has entered more than 10 formal letters of intent or definitive agreements and is negotiating more. It is the Company’s view that its expanding patent portfolio is a positive step in enabling the generation of more significant revenues during 2019.

| Page 42 of 83 |
| Table of Contents |
We do not expect that all of the Letters of Intent into which we enter will result in definitive agreements with paying customers and cannot predict how many will. We believe that strengthening and expanding our intellectual property portfolio and conducting supportive R&D will jointly contribute to strengthening revenue prospects.
General and Administrative
Our general and administrative expenses increased by $5,053,935 during the year ended August 31, 2018. The increase in our general and administrative expenses was largely due to non-cash expenses related to valuation of grants for service and share based payments required by contracts. The total of non-cash based payments for the period was $4,446,565.
If this non-cash expense is subtracted from the total expenses increase, then our G&A expenses increased by only $607,370. Contemplating the expenses other than the non-cash related items, actual cash expenses are in line with our expected increasing R&D, patent and trademark filings, and brand awareness requirements. The increases in executing budgeted work included significant increases in R&D for execution of studies supporting our patent filings, such as the in vivo Nicotine and European human studies, for a year on year increase of $438,679. Ongoing increases to legal expenses, year on year of $152,852 for our world-wide patent and trademark filings, as well as increases to our advertising and promotions to engage our markets to generate awareness and licensing clients, year on year $280,024. Fiscal 2019 expects to continue these increases based on available funding.
Interest Expense
Interest expense for the year ended August 31, 2018 was $Nil (2017 $6,015). The decrease was due to the conversion as of August 31, 2017 of the convertible debt and extinguishment of the long-term loan. The Company has no debt at this time other than month-to-month receivables.
Consulting fees
Our consulting fees increased during the year ended August 31, 2018 due to the involvement of additional consultants, contract updates and non-cash payments for services of $4,446,565. Our executives are typically hired and compensated as consultants and costs associated with those agreements comprise the majority of our consulting fees expense (Note 16) and thus our Consulting Expenses category includes certain fees that might otherwise be recognized under wages and salaries.
Professional Fees
Our professional fees increased by $164,318 to $289,062 during fiscal 2018 primarily due to increases in patent and trademark filings of $152,852, with the balance primarily being increases in tax and other accounting services. Although we always try to minimize expenses, we consider increases in costs related to patent and trademark work to reflect positive progress in executing our business plan. We recognize certain legal fees, tax advice fees, and accounting services all as “Professional Fees.”
Working Capital |
| August 31, 2018 $ |
|
| August 31, 2017 $ |
| ||
Current assets |
|
| 2,284,051 |
|
|
| 2,795,495 |
|
Current liabilities |
|
| 43,640 |
|
|
| 92,347 |
|
Net Working Capital |
|
| 2,240,411 |
|
|
| 2,703,148 |
|

| Page 43 of 83 |
| Table of Contents |
The Company’s working capital balance decrease during the year ended August 31, 2018, was limited due to the ongoing exercises of outstanding options and warrants providing significant incoming funds. The Company maintained a positive and strong working capital position throughout the year, which is slightly weaker at year-end.
|
| Year Ended |
| |||||
Cash Flows |
| August 31, 2018 $ |
|
| August 31, 2017 $ |
| ||
Cash flows (used in) provided by operating activities |
|
| (2,517,978 | ) |
|
| (1,545,909 | ) |
Cash flows (used in) provided by investing activities |
|
| (155,399 | ) |
|
| (9,699 | ) |
Cash flows (used in) provided by financing activities |
|
| 1,867,224 |
|
|
| 3,995,536 |
|
Increase (decrease) in cash |
|
| (806,153 | ) |
|
| 2,439,928 |
|
Operating Activities
Net cash used in operating activities was $2,514,332 for the year ended August 31, 2018 compared with cash used in operating activities of $1,545,909 during the same period in 2017. This difference was largely due to the increased costs pertaining to consulting, advertising and promotion, patent and trademark related filings, research and development, and travel.
Investing Activities
Net cash used in investing activities was $155,399 (2017 $9,699) for the year ended August 31, 2018 is due to the Company’s cost incurred related to its patent related applications $85,399 and the purchase of the remaining 49% of Poviva LLC of $70,000.
Financing Activities
Net cash provided from financing activities was $1,863,577 during the year ended August 31, 2018 compared to net cash provided of $3,995,536 during the same period in 2017. During fiscal 2018, the Company did not pursue additional financing, instead utilizing existing funding and ongoing exercises of stock options and warrant exercises only.
Going Concern
The Company’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (U.S. GAAP) applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has a net loss attributable to its common shareholders of $4,099,420 for the year ended August 31, 2019 (2018: $6,598,843) and at August 31, 2019 had a deficit accumulated since its inception of $23,868,202 (2018: $19,768,782). The Company has a working capital balance of $1,634,322 as at August 31, 2019 (2018: $2,240,411). The Company requires additional funds to maintain its operations and developments beyond fiscal 2020. Management’s plans in this regard are to raise equity and debt financing as required, but there is no certainty that such financing will be available or that it will be available at acceptable terms. The outcome of these matters cannot be predicted at this time.

| Page 44 of 83 |
| Table of Contents |
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Critical Accounting Policies
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with the accounting principles generally accepted in the United States of America (US GAAP). Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management’s application of accounting policies. We believe that understanding the basis and nature of the estimates and assumptions involved with the aspects of our financial statements are critical to an understanding of our financial statements as more particularly described in Note 3 to our audited annual consolidated financial statements included herein.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Not applicable. The Company qualifies as a “Smaller Reporting Company” and, accordingly, this Item and the related disclosure is not required.
Item 8. Financial Statements and Supplementary Data

| Page 45 of 83 |
| Table of Contents |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Directors of
Lexaria Bioscience Corp.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Lexaria Bioscience Corp. (the “Company”), as of August 31, 2019 and 2018, and the related consolidated statements of operations and comprehensive loss, cash flows, and stockholders’ equity for the years ended August 31, 2019 and 2018, and the related notes (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Lexaria Bioscience Corp. as of August 31, 2019 and 2018, and the results of its operations and its cash flows for the years ended August 31, 2019 and 2018 in conformity with accounting principles generally accepted in the United States of America.
Report on Internal Control Over Financial Reporting
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of August 31, 2019, based on the criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated November 13, 2019 expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting because of material weaknesses.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatements of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company’s auditor since 2016.
“DAVIDSON & COMPANY LLP”
Vancouver, Canada | Chartered Professional Accountants |
November 13, 2019 |
|
| Page 46 of 83 |
| Table of Contents |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Directors of
Lexaria Bioscience Corp.
Opinion on Internal Control over Financial Reporting
We have audited Lexaria Bioscience Corp.’s (the “Company”) internal control over financial reporting as of August 31, 2019, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, because of the effect of the material weaknesses identified below on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of August 31, 2019, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s consolidated balance sheet as of August 31, 2019, and the related consolidated statements of operations and comprehensive loss, cash flows, and stockholders’ equity for the year ended August 31, 2019, and the related notes and our report dated November 13, 2019 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.

| Page 47 of 83 |
| Table of Contents |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Material Weaknesses
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in management’s assessment.
Management did not design and maintain effective controls over the following, each of which is a material weakness:
(a) lack of adequate oversight related to the development and performance of internal controls;
(b) lack of defined policies and procedures to collect, process and act on whistleblower complaints; and
(c) lack of understanding and application of ASC 310 with respect to incurred losses.
These material weaknesses were considered in determining the nature, timing and extent of audit tests applied in our audit of the consolidated financial statements as of and for the year ended August 31, 2019, of the Company, and this report does not affect our report on such financial statements.
| “DAVIDSON & COMPANY LLP” |
|
|
Vancouver, Canada | Chartered Professional Accountants |
|
|
November 13, 2019 |
|
| Page 48 of 83 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP.
CONSOLIDATED BALANCE SHEET
(Expressed in U.S. Dollars)
|
| August 31 |
|
| August 31 |
| ||
|
| 2019 |
|
| 2018 |
| ||
|
| (Audited) |
|
| (Audited) |
| ||
ASSETS |
|
|
|
|
|
| ||
Current |
|
|
|
|
|
| ||
Cash and cash equivalents |
| $ | 1,285,147 |
|
| $ | 1,727,184 |
|
Marketable Securities (Note 21) |
|
| 64,214 |
|
|
| 10,151 |
|
Accounts receivable (Note 7) |
|
| 273,145 |
|
|
| 265,751 |
|
Inventory (Note 8) |
|
| 127,396 |
|
|
| 87,233 |
|
Prepaid expenses and deposit (Note 19) |
|
| 68,927 |
|
|
| 193,732 |
|
Total Current Assets |
|
| 1,818,829 |
|
|
| 2,284,051 |
|
|
|
|
|
|
|
|
|
|
Capital assets, net |
|
|
|
|
|
|
|
|
Patent (Note 9) |
|
| 265,127 |
|
|
| 146,538 |
|
Property & Equipment (Note 10) |
|
| 591,263 |
|
|
| 1,237 |
|
|
|
| 856,390 |
|
|
| 147,775 |
|
TOTAL ASSETS |
| $ | 2,675,219 |
|
| $ | 2,431,826 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES |
|
|
|
|
|
|
|
|
Current |
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities (Note 11) |
| $ | 136,411 |
|
| $ | 35,785 |
|
Due to a related party (Note 16) |
|
| 48,096 |
|
|
| 7,855 |
|
Total Current Liabilities |
|
| 184,507 |
|
|
| 43,640 |
|
TOTAL LIABILITIES |
|
| 184,507 |
|
|
| 43,640 |
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
Share Capital (Note 13) |
|
|
|
|
|
|
|
|
Authorized: |
|
|
|
|
|
|
|
|
220,000,000 common voting shares with a par value of $0.001 per share Issued and outstanding: 78,787,134 common shares at August 31, 2019 and 75,533,471 common shares at August 31, 2018 |
|
| 78,787 |
|
|
| 75,533 |
|
Additional paid-in capital (Note 13, 14) |
|
| 26,172,453 |
|
|
| 22,095,682 |
|
Accumulated Other Comprehensive Income |
|
| - |
|
|
| (14,247 | ) |
Deficit |
|
| (23,868,202 | ) |
|
| (19,768,782 | ) |
Equity attributable to shareholders of the Company |
|
| 2,383,038 |
|
|
| 2,388,186 |
|
Non-Controlling Interest |
|
| 107,674 |
|
|
| - |
|
Total Stockholders' Equity |
|
| 2,490,712 |
|
|
| 2,388,186 |
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
| $ | 2,675,219 |
|
| $ | 2,431,826 |
|
The accompanying notes are an integral party of these consolidated financial statements.

| Page 49 of 83 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Expressed in U.S. Dollars, except number of shares)
|
|
| YEAR ENDED |
| ||||||
|
|
| August 31 |
|
| August 31 |
| |||
|
|
| 2019 |
|
| 2018 |
| |||
Revenue (Note 15) |
|
| $ | 222,610 |
|
| $ | 433,287 |
| |
Cost of Goods Sold |
|
|
| 22,893 |
|
|
| 25,185 |
| |
Gross profit |
|
|
| 199,717 |
|
|
| 408,102 |
| |
|
|
|
|
|
|
|
|
|
| |
Expenses |
|
|
|
|
|
|
|
|
| |
Accounting and audit |
|
|
| 77,388 |
|
|
| 85,553 |
| |
Depreciation and amortization (Note 9, 10) |
|
|
| 60,550 |
|
|
| 2,307 |
| |
Advertising and promotions |
|
|
| 515,360 |
|
|
| 489,058 |
| |
Bad Debt |
|
|
| 75,000 |
|
|
| - |
| |
Consulting (Notes 13, 14, 16) |
|
|
| 1,444,735 |
|
|
| 5,332,398 |
| |
Investor relations |
|
|
| 203,893 |
|
|
| 188 |
| |
Legal and professional |
|
|
| 670,863 |
|
|
| 289,062 |
| |
Office and miscellaneous |
|
|
| 297,209 |
|
|
| 217,655 |
| |
Research and development |
|
|
| 555,730 |
|
|
| 492,864 |
| |
Travel |
|
|
| 100,587 |
|
|
| 99,236 |
| |
Wages & Salaries |
|
|
| 333,199 |
|
|
| - |
| |
Gain on disposal of assets |
|
|
| - |
|
|
| (3,998 | ) | |
Unrealized Loss on marketable securities (Note 21) |
|
|
| 16,434 |
|
|
| - |
| |
Inventory writeoff (Note 8) |
|
|
| 7,182 |
|
|
| 12,966 |
| |
|
|
|
| 4,358,130 |
|
|
| 7,017,289 |
| |
|
|
|
|
|
|
|
|
|
| |
Net (loss) and comprehensive loss for the period |
|
| $ | (4,158,413 | ) |
| $ | (6,609,187 | ) | |
Net (loss) and comprehensive loss attributable to: |
|
|
|
|
|
|
|
|
| |
Common Shareholders |
|
| $ | (4,099,420 | ) |
| $ | (6,598,843 | ) | |
Non-Controlling Interest |
|
| $ | (58,993 | ) |
| $ | (10,344 | ) | |
|
|
|
|
|
|
|
|
|
| |
Basic and diluted (loss) per share |
|
| $ | (0.05 | ) |
| $ | (0.09 | ) | |
|
|
|
|
|
|
|
|
|
| |
Weighted average number of common shares outstanding |
|
|
|
|
|
|
|
|
| |
-Basic and diluted |
|
|
| 77,792,263 |
|
|
| 70,960,416 |
| |
The accompanying notes are an integral part of these audited consolidated financial statements.

| Page 50 of 83 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP.
CONSOLIDATED STATEMENT OF CASH FLOWS
(Expressed in U.S. Dollars)
|
| YEAR ENDED |
| |||||
|
| August 31 |
|
| August 31 |
| ||
|
| 2019 |
|
| 2018 |
| ||
Cash flows used in operating activities |
|
|
|
|
|
| ||
Net loss and comprehensive loss |
| $ | (4,158,413 | ) |
| $ | (6,609,187 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Stock based compensation |
|
| 626,692 |
|
|
| 2,602,239 |
|
Depreciation and amortization |
|
| 60,550 |
|
|
| 2,307 |
|
Inventory write-off (Note 8) |
|
| 7,182 |
|
|
| 12,966 |
|
Bad Debt Expense |
|
| 75,000 |
|
|
| - |
|
Unrealized loss on marketable securities |
|
| 16,434 |
|
|
| - |
|
Unrealized foreign exchange |
|
| - |
|
|
| 602 |
|
Common shares issued for services |
|
| 234,500 |
|
|
| 781,056 |
|
Warrants issued for services |
|
| 52,817 |
|
|
| 1,063,270 |
|
Change in working capital |
|
|
|
|
|
|
|
|
Accounts receivable |
|
| (138,644 | ) |
|
| (245,458 | ) |
Inventory |
|
| (47,345 | ) |
|
| (33,025 | ) |
Prepaid expenses and deposits |
|
| 124,805 |
|
|
| (44,041 | ) |
Accounts payable and accrued liabilities |
|
| 100,626 |
|
|
| 3,210 |
|
Due to related parties |
|
| 40,241 |
|
|
| (34,835 | ) |
Deferred revenue |
|
| - |
|
|
| (17,083 | ) |
Net cash used in operating activities |
| $ | (3,005,555 | ) |
| $ | (2,517,979 | ) |
|
|
|
|
|
|
|
|
|
Cash flows used in investing activities |
|
|
|
|
|
|
|
|
Investment in Poviva |
|
| - |
|
|
| (70,000 | ) |
Patent |
|
| (122,982 | ) |
|
| (85,399 | ) |
Property & Equipment |
|
| (646,183 | ) |
|
| - |
|
Net cash used in investing activities |
| $ | (769,165 | ) |
| $ | (155,399 | ) |
|
|
|
|
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
Investment from NCI |
|
| 1,000,000 |
|
|
| - |
|
Proceeds from issuance of equity |
|
| 2,332,683 |
|
|
| 1,867,224 |
|
Net cash from financing activities |
| $ | 3,332,683 |
|
| $ | 1,867,224 |
|
|
|
|
|
|
|
|
|
|
Decrease in cash and cash equivalents |
|
| (442,037 | ) |
|
| (806,153 | ) |
Cash and cash equivalents, beginning of year |
|
| 1,727,184 |
|
|
| 2,533,337 |
|
Cash and cash equivalents, end of year |
| $ | 1,285,147 |
|
| $ | 1,727,184 |
|
|
|
|
|
|
|
|
|
|
Supplemental information of cash flows: |
|
|
|
|
|
|
|
|
Income taxes paid in cash |
| $ | 13,919 |
|
| $ | - |
|
Common shares issued to settle AP |
| $ | - |
|
| $ | 12,000 |
|
Reclassification of NCI to additional paid in capital on acquisition |
| $ | 833,333 |
|
| $ | 318,820 |
|
The accompanying notes are an integral part of these audited consolidated financial statements.

| Page 51 of 83 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS EQUITY
(Expressed in U.S. Dollars)
|
| COMMON STOCK |
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||
|
| SHARES |
|
| AMOUNT $ |
|
| ADDITIONAL PAID-IN CAPITAL $ |
|
| DEFICIT $ |
|
| NCI $ |
|
| AOCI $ |
|
| TOTAL STOCKHOLDERS EQUITY $ |
| |||||||
Balance August 31, 2017 |
|
| 67,975,761 |
|
|
| 67,976 |
|
|
| 16,108,270 |
|
|
| (13,169,939 | ) |
|
| (238,476 | ) |
|
| - |
|
|
| 2,767,831 |
|
Non-controlling interest |
|
| - |
|
|
| - |
|
|
| (318,820 | ) |
|
| - |
|
|
| 248,820 |
|
|
| - |
|
|
| (70,000 | ) |
Shares issued for services |
|
| 647,690 |
|
|
| 648 |
|
|
| 780,408 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 781,056 |
|
Stock based compensation |
|
| - |
|
|
| - |
|
|
| 2,602,239 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 2,602,239 |
|
Warrants issued for services |
|
| - |
|
|
| - |
|
|
| 1,063,270 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,063,270 |
|
Exercise of stock options |
|
| 545,875 |
|
|
| 546 |
|
|
| 93,156 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 93,702 |
|
Exercise of warrants |
|
| 6,364,145 |
|
|
| 6,363 |
|
|
| 1,767,159 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,773,522 |
|
Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (6,598,843 | ) |
|
| (10,344 | ) |
|
| - |
|
|
| (6,609,187 | ) |
Other comprehensive loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (14,247 | ) |
|
| (14,247 | ) |
Balance August 31, 2018 |
|
| 75,533,471 |
|
|
| 75,533 |
|
|
| 22,095,682 |
|
|
| (19,768,782 | ) |
|
| - |
|
|
| (14,247 | ) |
|
| 2,388,186 |
|
Shares issued for services |
|
| 250,000 |
|
|
| 250 |
|
|
| 234,250 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 234,500 |
|
Stock based compensation |
|
| - |
|
|
| - |
|
|
| 626,692 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 626,692 |
|
Warrants issued for services |
|
| - |
|
|
| - |
|
|
| 52,817 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 52,817 |
|
Exercise of stock options |
|
| 430,000 |
|
|
| 430 |
|
|
| 65,820 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 66,250 |
|
Exercise of warrants |
|
| 1,626,513 |
|
|
| 1,627 |
|
|
| 794,496 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 796,123 |
|
Private Placement |
|
| 947,150 |
|
|
| 947 |
|
|
| 1,469,363 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,470,310 |
|
Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (4,099,420 | ) |
|
| - |
|
|
| - |
|
|
| (4,099,420 | ) |
Non-controlling interest |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (58,993 | ) |
|
| - |
|
|
| (58,993 | ) |
Other comprehensive income |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 14,247 |
|
|
| 14,247 |
|
Subsidiary Investment |
|
| - |
|
|
| - |
|
|
| 833,333 |
|
|
| - |
|
|
| 166,667 |
|
|
| - |
|
|
| 1,000,000 |
|
Balance August 31, 2019 |
|
| 78,787,134 |
|
|
| 78,787 |
|
|
| 26,172,453 |
|
|
| (23,868,202 | ) |
|
| 107,674 |
|
|
| - |
|
|
| 2,490,712 |
|
The accompanying notes are an integral part of these audited consolidated financial statements.

| Page 52 of 83 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
August 31, 2019
(Expressed in U.S. Dollars)
| 1. | Organization, Business and Going Concern |
|
|
| Lexaria Bioscience Corp. (“Lexaria”, or the “Company”) was formed on December 9, 2004 under the laws of the State of Nevada. In March of 2014, the Company began its entry into the bioscience and alternative health and wellness business and in May 2016, the Company commenced out-licensing its patented DehydraTECH™ technology (the “Technology”) for improved delivery of bioactive compounds that promotes healthy ingestion methods, lower overall dosing and higher effectiveness in active molecule delivery. The Company has its office in Kelowna, BC, Canada. | |
|
|
| The Company’s consolidated financial statements included herein have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission and in accordance with accounting principles generally accepted in the United States (US GAAP) applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The recurring losses from operations and net capital deficiency raise substantial doubt about the Company’s ability to continue as a going concern. | |
|
|
| The Company requires additional funds to maintain its operations and developments. Management’s plans in this regard are to raise equity and debt financing as required, but there is no certainty that such financing will be available or that it will be available at acceptable terms. The outcome of these matters cannot be predicted at this time. | |
|
|
| 2. | Business Risk and Liquidity |
|
|
| The Company is subject to several categories of risk associated with its operating activities. The production and sale of alternative health products is an emerging industry in which business practices are not yet standardized and are subject to frequent scrutiny and evaluation by federal, state, provincial, and municipal authorities, academics, and media outlets, among others. Although we intend to develop our businesses in accordance with best ethical practices, we may suffer negative publicity if we, our partners, contractors, or customers are found to have engaged in any environmentally insensitive practices or other business practices that are viewed as unethical. | |
|
|
| Our operations may require licenses and permits from various governmental authorities. We believe that we will be able to obtain all necessary licenses and permits under applicable laws and regulations for our operations and believe we will be able to comply in all material respects with the terms of such licenses and permits. However, such licenses and permits are subject to change in various circumstances. There can be no guarantee that we will be able to obtain or maintain all necessary licenses and permits, and failing to obtain or retain required licenses could have a materially adverse effect on the Company. | |
|
|
| Lexaria and its subsidiaries are not involved directly or indirectly in the cultivation, processing, distribution, or utilization of Cannabis or Cannabis derived components. All of Lexaria’s consumer products utilize legally sourced Hemp and Hemp components in their production. Lexaria does have an ancillary involvement risk via out-licensing of its patented Technology to licensees that choose to utilize its Technology to manufacture products that contain locally or state approved but federally regulated and controlled contents. There can be no guarantee that changes in the regulatory framework and environment will not occur and such changes could have a materially adverse effect on the Company. | |
|
|
| Lexaria and its subsidiaries are not involved directly or indirectly in the production or sale of any products containing nicotine. Products containing nicotine have historically been involved in litigation in the USA. Lexaria’s corporate licensee may introduce products containing nicotine that utilize Lexaria’s technology to the US consumer market, which could therefore introduce third-party risks to Lexaria. |

| Page 53 of 83 |
| Table of Contents |
| 3. | Significant Accounting Policies |
|
|
| a) Accounting Principles | |
|
|
| These consolidated financial statements have been prepared in conformity with generally accepted accounting principles of the United States of America. All amounts, unless otherwise stated, are in United States dollars. | |
|
|
| b) Revenue Recognition | |
|
|
| Product Revenue | |
|
|
| Revenue from the sale of products is recognized when persuasive evidence of an arrangement exists, delivery has occurred, the sales price is fixed or determinable, and collectability is reasonably assured, which typically occurs upon shipment. The Company reports its sales net of the amount of actual sales returns. Sales tax collected from customers is excluded from net sales. | |
|
|
| Licensing Revenue from Intellectual Property | |
|
|
| We recognize revenue for License fees at a point in time following the transfer of our intellectual property, our patented lipid nutrient infusion technology DehydraTECH™ for infusing Active Pharmaceutical Ingredients, to the licensee, which typically occurs on delivery of documentation. | |
|
|
| Usage Fees from Intellectual Property | |
|
|
| We recognize revenue for Usage fees when usage of our DehydraTECH intellectual property occurs by licensees infusing an Active Pharmaceutical Ingredient into one or more of their product lines for sale. | |
|
|
| c) Inventory and Cost of Sales | |
|
|
| The Company’s inventory consists of finished goods, work in progress, and raw materials. In all classes, inventory is valued at the lower of cost or market. Cost is determined on a first-in, first-out basis. | |
|
|
| Cost of sales includes all expenditures incurred in bringing the goods to the point of sale. Inventory costs and costs of sales include direct costs of the raw material, inbound freight charges, warehousing costs, handling costs (receiving and purchasing) and utilities and overhead expenses related to the Company’s manufacturing and processing facilities. | |
|
|
| d) Cash and Cash Equivalents | |
|
|
| Cash equivalents comprise certain highly liquid instruments with a maturity of three months or less when purchased. As of August 31, 2019, and August 31, 2018, the Company held cash only. | |
|
|
| e) Equipment | |
|
|
| Equipment is stated at cost less accumulated depreciation and impairment, and depreciated using the straight-line method over their useful lives or by units of production. | |
|
|
| f) Patents | |
|
|
| Capitalized patent costs represent legal costs incurred to establish patents. When patents reach a mature stage, any associated legal costs are comprised mostly of maintenance fees and are expensed as incurred. Capitalized patent costs are amortized on a straight-line basis over the remaining life of the patent. The Company was granted its first patent on October 25, 2016, with a legal life of 20 years. Additional patent information is in Note 9. |

| Page 54 of 83 |
| Table of Contents |
| g) Stock-Based Compensation | |
|
|
| The Company accounts for its stock-based compensation awards in accordance with ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees, including grants of employee stock options, to be recognized as expenses in the statements of operations based on their grant date fair values. For stock options granted to employees and to members of the Board of Directors for their services on the Board of Directors, the Company estimates the grant date fair value of each option award using the Black-Scholes option-pricing model. The use of the Black-Scholes option-pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk-free interest rates and expected dividend yields of the common stock. | |
|
|
| Stock-based payments issued to non-employees are recorded at their fair values and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period in accordance with the provisions of ASC 718 and ASC Topic 505, Equity. For equity instruments granted to non-employees, the Company recognizes stock-based compensation expense on vesting. | |
|
|
| h) Loss Per Share
The Company applies the guidance in ASC 260 Earnings Per Share. Loss per share is computed using the weighted average number of shares outstanding during the period. Diluted loss per share is equivalent to basic loss per share because the potential exercise of the equity-based financial instruments was anti-dilutive.
i) Foreign Currency Translation
The Company s operations are located in the United States of America and Canada, and it has offices in Canada. The Company maintains its accounting records in U.S. Dollars, as follows:
At the transaction date, each asset, liability, revenue and expense that was acquired or incurred in a foreign currency is translated into U.S. dollars by using the exchange rate in effect at that date. At the period end, monetary assets and liabilities are translated at the exchange rate in effect at that date. The resulting foreign exchange gains and losses are included in profit or loss.
j) Financial Instruments
ASC 820 Fair Value Measurements and Disclosures, requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1 - Quoted prices in active markets for identical assets or liabilities;
Level 2 - Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable; and
Level 3 - Unobservable inputs that are supported by little or no market activity, therefore requiring an entity to develop its own assumptions about the assumptions that market participants would use in pricing.
The Company s financial instruments consist primarily of cash, marketable securities, accounts receivable, accounts payable and accrued liabilities, and due to related parties. The carrying amounts of cash, accounts and other receivable, accounts payable and accrued liabilities, and due to related parties approximate their fair values due to their short maturities or quoted market prices. |

| Page 55 of 83 |
| Table of Contents |
| The Company is located in Canada, which results in exposure to market risks from changes in foreign currency rates. The foreign currency exchange risk is the financial risk to the Company’s operations that arise from fluctuations in foreign exchange rates and the degree of volatility of these rates. Currently, the Company does not use derivative instruments to reduce its exposure to foreign currency risk as the Company does not hold a significant position in foreign currencies, such as the Canadian dollar, and the impact of a change in a few basis points for USD/CAD is not expected to be material. | |
|
|
| k) Income Taxes | |
|
|
| The Company applies the guidance in ASC 740, Income Taxes, which requires the Company to recognize deferred tax liabilities and assets for the expected future tax consequences of events that have been recognized in the Company’s financial statements or tax returns using the liability method. Under this method, deferred tax liabilities and assets are determined based on the temporary differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect in the year in which the differences are expected to reverse. | |
|
|
| l) Impairment of Long-Lived Assets
Long-lived assets, including equipment, and intangible assets, such as the Company s patents, are assessed for potential impairment when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. An impairment loss is recognized when the carrying amount of the long-lived asset is not recoverable and exceeds its fair value. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. Any required impairment loss is measured as the amount by which the carrying amount of the long-lived asset exceeds its fair value and is recorded as a reduction in the carrying value of the related asset and a charge to the profit or loss. Intangible assets with indefinite lives are tested for impairment annually and in interim periods if certain events occur indicating that the carrying value of the intangible assets may be impaired.
m) Comprehensive Income
The Company applies ASC 220, Comprehensive Income, which establishes standards for reporting and presentation of comprehensive income, its components and accumulated balances. The Company discloses this information on its Statement of Stockholders Equity. Comprehensive income comprises equity changes except those transactions resulting from investments by owners and distributions to owners.
n) Credit Risk and Receivable Concentration
The Company places its cash with a high credit quality financial institution. As of August 31, 2019, the Company had approximately $1,285,147 in the bank (August 31, 2018: $1,727,184).
As at August 31, 2019 we had $106,000 (2018 $199,375) in Intellectual Property Territory License fees receivable (Note 7) consisting of amounts due from three licensees (2018 three). These receivable amounts are based on contractual terms for payments that are payable within twelve months of signing the definitive agreements or routine IP Usage Fees. To date these licensees have performed all of their required obligations. The Company incurred $75,000 in bad debt in fiscal 2019.
As at August 31, 2019, the Company had $161,418 (2018 - $61,176) in sales tax receivable (Note 6). The Company considers its credit risk to be low for such receivables.
o) Commitments and Contingencies
In accordance with ASC 450-20, Accounting for Contingencies, the Company records accruals for such loss contingencies when it is probable that a liability has been incurred and the amount of loss can be reasonably estimated. In the event that estimates or assumptions prove to differ from actual results, adjustments are made in subsequent periods to reflect more current information. Historically, the Company has not experienced any material claims. |

| Page 56 of 83 |
| Table of Contents |
| p) Research and Development | |
|
|
| Research and development costs are expensed as incurred. |
| 4. | Basis of Consolidation |
|
|
| These consolidated financial statements include the financial statements of the Company and its wholly owned subsidiaries; Lexaria CanPharm ULC, PoViva Corp., Lexaria Hemp Corp., Kelowna Management Services Corp. and Lexaria Pharmaceutical Corp, and our subsidiary Lexaria Nicotine LLC. On January 15, 2019, the Company announced the initial investment of $1,000,000 from Altria Ventures Inc., an indirect wholly owned subsidiary of Altria Group, Inc., for a 16.667% equity interest along with certain other rights in Lexaria Nicotine LLC. All significant intercompany balances and transactions have been eliminated. | |
|
|
| 5. | Estimates and Judgments |
|
|
| The preparation of financial statements in conformity with U.S GAAP requires us to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Some of the Company’s accounting policies require us to make subjective judgments, often as a result of the need to make estimates of matters that are inherently uncertain. These accounting policies involve critical accounting estimates because they are particularly dependent on estimates and assumptions made by management about matters that are highly uncertain at the time the accounting estimates are made. Although we have used our best estimates based on facts and circumstances available to us at the time, different estimates reasonably could have been used. Changes in the accounting estimates used by the Company are reasonably likely to occur from time to time, which may have a material effect on the presentation of financial condition and results of operations. | |
|
|
| The Company reviews these estimates, judgments and assumptions periodically and reflect the effects of revisions in the period in which they are deemed to be necessary. We believe that these estimates are reasonable; however, actual results could differ from these estimates. | |
|
|
| Significant accounting estimates and assumptions are used for, but not limited to: | |
|
|
| a) The Valuation of Deferred Tax Assets
Judgement is required in determining whether deferred tax assets are recognized on the balance sheet. The recognition of deferred tax assets requires management to assess the likelihood that the Company will generate taxable income in future periods to utilize the deferred tax assets. Due to the Company s history of losses, deferred tax assets have not been recognized by Lexaria.
b) Value of Stock Options and Warrants
The Company provides compensation benefits to its employees, directors, officers, and consultants, through a stock option plan. The fair value of each option award is estimated on the date of grant using the Black-Scholes option pricing model. Expected volatility assumptions used in the model is based on the historical volatility of the Company s share price. The Company uses historical data to estimate the period of option exercises for use in the valuation model. The risk-free interest rate for the expected term of the option is based on the yields of government bonds. Changes in these assumptions, especially the share price volatility and the expected life determination could have a material impact on the Company s profit and loss for the periods presented. All estimates used in the model are based on historical data which may not be representative of future results. |

| Page 57 of 83 |
| Table of Contents |
| 6. | Recent Accounting Guidance |
|
|
| In January 2016, FASB issued an ASU, Subtopic 82510, to amend certain aspects of recognition, measurement, presentation, and disclosure of financial instruments. Most prominent among the amendments is the requirement for changes in fair value of equity investments, with certain exceptions, to be recognized through profit or loss rather than other comprehensive income. The Company adopted the standard September 1, 2018. The impact was not material and the $14,247 impact on the Company’s financial statements was included in income in the current period. | |
|
|
| In February 2016 FASB issued ASU No. 201602, Leases (Topic 842) which supersedes FASB ASC Topic 840, Leases (Topic 840) and provides principles for the recognition, measurement, presentation, and disclosure of leases for both lessees and the lessors. The new standard requires the lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. The classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease, respectively. A lessee is also required to record a right of use asset and a lease liability for all leases with a term of greater than twelve months regardless of classification. Leases with a term of twelve months or less will be accounted for similar to existing guidance for operating leases. The standard is effective for annual and interim periods beginning after December 15, 2018, with early adoption permitted upon issuance. When adopted, the Company does not expect this guidance to have a material impact on its consolidated financial statements. | |
|
|
| In June 2016, the FASB issued a new standard to replace the incurred loss impairment methodology in current U.S. GAAP with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. For trade and other receivables, loans and other financial instruments, the Company will be required to use a forward-looking expected loss model rather than the incurred loss model for recognizing credit losses which reflects losses that are probable. Credit losses relating to available for sale debt securities will also be recorded through an allowance for credit losses rather than as a reduction in the amortized cost basis of the securities. The new standard will be effective for Lexaria beginning September 1, 2020, with early adoption permitted. Application of the amendments is through a cumulative effect adjustment to deficit as of the effective date. The Company is currently assessing the impact of the standard on its consolidated financial statements. | |
|
|
| In February 2018, the FASB issued ASU No. 201802, Income Statement–Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income, which allows a reclassification from accumulated other comprehensive income to retained earnings for stranded tax effects resulting from the Tax Cuts and Jobs Act enacted by the U.S. federal government on December 22, 2017 (the “2017 Tax Act”). Consequently, the amendments eliminate the stranded tax effects resulting from the 2017 Tax Act and will improve the usefulness of information reported to financial statement users. The amendments in this ASU are effective for all entities for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted, including adoption in any interim period, (1) for public business entities for reporting periods for which financial statements have not yet been issued and (2) for all other entities for reporting periods for which financial statements have not yet been made available for issuance. The Company is currently evaluating the effect this ASU will have on its consolidated financial statements and related disclosures but does not expect it to have a material impact on its consolidated financial statements. | |
|
|
| In June 2018, the FASB issued ASU No. 201807, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share Based Payment Accounting. This is a simplification that involves several aspects of accounting for nonemployee share-based payments resulting from expanding the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. The new standard will be effective for Lexaria for September 1, 2019. The Company does not expect it to have a material impact on its consolidated financial statements. |

| Page 58 of 83 |
| Table of Contents |
7. Accounts and Other Receivables
|
| August 31 |
|
| August 31 |
| ||
|
| 2019 |
|
| 2018 |
| ||
|
| $ |
|
| $ |
| ||
Trade and deposits receivable |
|
| 5,727 |
|
|
| 5,200 |
|
Territory License Fee receivable |
|
| 106,000 |
|
|
| 199,375 |
|
Sales tax receivable |
|
| 161,418 |
|
|
| 61,176 |
|
|
|
| 273,145 |
|
|
| 265,751 |
|
| 8. | Inventory |
|
| August 31 |
|
| August 31 |
| ||
|
| 2019 |
|
| 2018 |
| ||
|
| $ |
|
| $ |
| ||
Raw materials |
|
| 45,068 |
|
|
| 29,355 |
|
Work in progress |
|
| - |
|
|
| 9,752 |
|
Finished goods |
|
| 82,328 |
|
|
| 48,126 |
|
|
|
| 127,396 |
|
|
| 87,233 |
|
| During the year ended August 31, 2019, the Company wrote down $7,182 (2018 - $12,966) of inventory to reflect its net realisable value. | |
|
|
| 9. | Intellectual Property |
|
|
| On November 12, 2014, the Company signed an agreement with Poppy’s Teas LLC. whereby it acquired a 51% interest. Subsequent to signing the agreement, Poppy’s Teas LLC effected a name change to PoViva Tea LLC. The Company acquired the remaining 49% ownership interest in PoViva Tea, LLC in October 2017 via compensation of $70,000, a waiver on certain debts owed to Lexaria, and a 5%, 20-year royalty on net profits of ViPova TeaTM tea, coffee, and hot chocolate sales. No Lexaria stock or options were issued. On September 18, 2018 Poviva Tea, LLC converted from a Nevada limited liability company to a Nevada corporation and effected a name change to Poviva Corp. | |
|
|
| The following is a list of US capitalized patents held by the Company |
Issued Patent # | Patent Issuance Date | Patent Family |
US 9,474,725 B1 | 10/25/2016 | Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof
|
US 9,839,612 B2 | 12/12/2017 | |
US 9,972,680 B2 | 05/15/2018 | |
US 9,974,739 B2 | 05/22/2018 | |
US 10,084,044 B2 | 09/25/2018 | |
US 10,103,225 B2 | 10/16/2018 | |
US 10,381,440 | 08/13/2019 | |
US 10,374,036 | 08/06/2019 |

| Page 59 of 83 |
| Table of Contents |
| The Company also holds non-capitalized patents outside the US. A continuity schedule for patents is presented below: |
|
| August 31 |
|
| August 31 |
| ||
|
| 2019 |
|
| 2018 |
| ||
|
| $ |
|
| $ |
| ||
Balance – Beginning |
|
| 146,538 |
|
|
| 62,827 |
|
Addition |
|
| 122,982 |
|
|
| 85,399 |
|
Amortization* |
|
| (4,393 | ) |
|
| (1,688 | ) |
Balance – Ending |
|
| 265,127 |
|
|
| 146,538 |
|
* The patents are amortized over their legal life of 20 years. | ||||||||
10. Property & Equipment
|
| Cost |
|
| Period Amortization |
|
| Accumulated Amortization |
|
| Net Balance August 31, 2018 |
| ||||
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
Equipment |
|
| 3,094 |
|
|
| (619 | ) |
|
| (1,857 | ) |
|
| 1,237 |
|
|
|
| 3,094 |
|
|
| (619 | ) |
|
| (1,857 | ) |
|
| 1,237 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cost |
|
| Period Amortization |
|
| Accumulated Amortization |
|
| Net Balance August 31, 2019 |
| ||||
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
Leasehold improvements |
|
| 259,981 |
|
|
| (33,342 | ) |
|
| (33,342 | ) |
|
| 226,639 |
|
Computers |
|
| 63,964 |
|
|
| (12,187 | ) |
|
| (12,187 | ) |
|
| 51,777 |
|
Furniture fixtures equipment |
|
| 34,220 |
|
|
| (4,205 | ) |
|
| (6,062 | ) |
|
| 28,158 |
|
Lab equipment |
|
| 291,235 |
|
|
| (6,546 | ) |
|
| (6,546 | ) |
|
| 284,689 |
|
|
|
| 649,400 |
|
|
| (56,281 | ) |
|
| (58,137 | ) |
|
| 591,263 |
|
11. Accounts Payable and Accrued Liabilities
|
| August 31 |
|
| August 31 |
| ||
|
| 2019 |
|
| 2018 |
| ||
|
| $ |
|
| $ |
| ||
Accounts Payable |
|
|
|
|
|
| ||
Trades Payable |
|
| 31,463 |
|
|
| 14,378 |
|
Sales Tax Payable |
|
| 63,616 |
|
|
| 1,869 |
|
Accrued Liabilities |
|
|
|
|
|
|
|
|
Trades Payable |
|
| 41,332 |
|
|
| 19,538 |
|
Balance – Ending |
|
| 136,411 |
|
|
| 35,785 |
|
| 12. | Unearned Revenue |
|
|
| On May 14, 2016, the Company entered into a licensing agreement (the “Licensing Agreement”) with an arm’s length party (the “Licensee”) allowing the Licensee, for a two-year period, to utilize the Company’s Technology to create, test, manufacture, and sell marijuana-infused consumable and/or topical products, in the state of Colorado, with an option of extending the terms of the Licensing Agreement to Washington, Oregon, and California (the “Territorial License”). In addition to the granting of the license, the Company is required to provide support services to the Licensee in connection with the use of the Company’s Technology during the term of the Licensing Agreement. |

| Page 60 of 83 |
| Table of Contents |
| The Company determined that the provision of the support services were a separate deliverable under the licensing agreement. Accordingly, the Company recognized revenue on a pro-rated basis over the term of the Licensing agreement. The Company has since determined that the support services form an insignificant portion of the licensing contract as they are primarily completed prior to delivery of the technology and that delivery of the license is complete when the Technology is transferred to the Licensee. During the year ended August 31, 2019, the Company recognized $Nil (2018 $17,083) (Note 14), of unearned revenue. |
|
| August 31 2019 $ |
|
| August 31 2018 $ |
| ||
Balance – Beginning |
|
| - |
|
|
| 17,083 |
|
Earned revenue (Note 15) |
|
| - |
|
|
| (17,083 | ) |
Balance – Ending |
|
| - |
|
|
| - |
|
| 13. | Common Shares and Warrants |
|
|
| Fiscal 2019 Activity
During the year ended August 31, 2019 the Company closed a non-brokered private placement for 947,150 Units priced at $1.60 each. Each Unit consists of one common share and one Share purchase warrant. Each warrant shall entitle the holder to acquire one common share at a price of $2.25 per Share for a period of 24 months. The Company also issued 28,175 broker warrants. The broker warrants have a term of 24 months and are each exercisable into one common share of the Company at a price of $2.25. The fair value of these broker warrants was determined to be $16,095, which were recorded as a share issue cost within additional paid in capital for a net effect of $Nil.
The company granted a total of 107,737 broker warrants with a value of $6,484 that were recorded as a share issue cost within additional paid in capital for a net effect of $Nil.
The company granted a total of 100,00 warrants pursuant to an agreement with a vendor valued at $52,817 that were recorded as an expense within investor relations.
During the year ended August 31, 2018 the Company recognized $51,448 in consulting expense for warrants previously granted to a consultant upon vesting.
Fiscal 2018 Activity
On October 27, 2017 the Company extended the expiration date of warrants originally issued on January 9, 2017, with a one-year expiration date. The warrant quantity and exercise price remain unchanged, 500,000 warrants exercisable at $0.44, will now expire on January 9, 2019. There was a $Nil effect on the modification of the warrants.
During the year ended August 31, 2018 the Company granted a total of 1,000,000 warrants with a fair value of $1,011,822 pursuant to consulting agreements signed during the year. The value of the warrants was recorded in consulting fees on the statement of operations. The company also granted a total of 35,913 warrants with a value of $21,646 which were recorded as a share issue cost within additional paid in capital for a net effect of $Nil.
During the year ended August 31, 2018 the Company recognized $51,448 in consulting expense for warrants previously granted to a consultant upon vesting. |

| Page 61 of 83 |
| Table of Contents |
| A summary of share issuance is presented relating to option and warrant exercises, agreement requirements and debt settlement is presented below: |
Type of Issuance |
| Number of Shares |
|
| Total Value |
| ||
Warrant exercise(1) |
|
| 1,626,513 |
|
|
| 796,122 |
|
Option exercise |
|
| 430,000 |
|
|
| 66,250 |
|
Private placement |
|
| 947,150 |
|
|
| 1,515,440 |
|
Per agreements(2) |
|
| 250,000 |
|
|
| 234,500 |
|
|
|
| 3,253,663 |
|
| $ | 2,612,312 |
|
| (1) Includes 384,212 broker warrants exercised for gross proceeds of $191,742 | |
| (2) The Company awarded the restricted common shares as required by consulting contracts | |
| A continuity schedule for warrants is presented below: |
|
| Number of Warrants |
|
| Weighted Average Exercise Price $ |
| ||
Balance August 31, 2017 |
|
| 8,844,506 |
|
|
| 0.29 |
|
Cancelled/Expired |
|
| (230,000 | ) |
|
| 0.17 |
|
Exercised |
|
| (6,364,145 | ) |
|
| 0.28 |
|
Issued |
|
| 1,035,913 |
|
|
| 1.48 |
|
Balance August 31, 2018 |
|
| 3,286,274 |
|
|
| 0.72 |
|
Cancelled/Expired |
|
| (17,498 | ) |
|
| 0.59 |
|
Exercised |
|
| (1,626,513 | ) |
|
| 0.49 |
|
Issued |
|
| 1,183,062 |
|
|
| 1.99 |
|
Balance August 31, 2019 |
|
| 2,825,325 |
|
|
| 1.38 |
|
| The fair value of share purchase warrants granted as broker warrants, compensation units, and compensatory warrants, was estimated as of the date of the grant by using the Black-Scholes option pricing model with the following assumptions: |
|
| August 31 2019 |
| August 31 2018 |
Expected volatility |
| 1% – 117% |
| 100% – 154% |
Risk-free interest rate |
| 2.31% – 2.87% |
| 1.21% – 2.60% |
Expected life |
| 1 day – 2 years |
| 1.21 – 3 years |
Dividend yield |
| 0.00% |
| 0.00% |
Estimated fair value per warrant |
| $Nil – $0.57 |
| $0.40 – $1.48 |
| A summary of warrants outstanding as of August 31, 2019 is presented below: |
# of Warrants |
|
| Weighted Average Remaining Contractual Life |
| Weighted Average Exercise Price $ |
| ||
| 250,000 |
|
| 0.25 years |
|
| 0.83 |
|
| 500,000 |
|
| 0.38 years |
|
| 1.83 |
|
| 975,325 |
|
| 1.17 years |
|
| 2.25 |
|
| 100,000 |
|
| 1.73 years |
|
| 0.96 |
|
| 250,000 |
|
| 1.73 years |
|
| 1.55 |
|
| 750,000 |
|
| 2.11 years |
|
| 0.14 |
|
| 2,825,325 |
|
| 1.27 years |
|
| 1.38 |
|

| Page 62 of 83 |
| Table of Contents |
Fiscal 2019 Activity The Company granted in the period ending August 31, 2019:14. Stock Options The Company has established its 2007 Equity Incentive Plan, whereby the board of directors may grant up to 412,500 stock options to eligible employees and directors, the 2010 Stock Option Plan whereby the board of directors may, from time to time, grant up to 1,512,500 stock options to officers and employees, and its 2014 Stock Option Plan whereby the board of directors may, from time to time, grant up to 2,107,500 stock options to directors, officers, employees, and consultants, the Equity Incentive Plan whereby the board of directors may, from time to time, grant up to 7,838,713 stock options to directors, officers, employees, and consultants. Stock options granted must be exercised no later than five years from the date of grant or such lesser period as determined by the Company’s board of directors. The exercise price of an option is equal to or greater than the closing market price of the Company’s common shares on the day preceding the date of grant. The vesting terms of each grant are set by the board of directors.
Quantity |
|
| Exercise Price $ |
|
| Life (Years) |
| |||
| 390,000(1) |
|
| 1.27 |
|
|
| 5 |
| |
| 240,000(1) |
|
| 1.06 |
|
|
| 5 |
| |
| 30,000(1) |
|
| 1.16 |
|
|
| 5 |
| |
| 350,000 |
|
|
| 0.99 |
|
|
| 5 |
|
| 440,000(1) |
|
| 0.99 |
|
|
| 5 |
| |
| 48,000(1) |
|
| 0.96 |
|
|
| 5 |
| |
| 100,000 |
|
|
| 0.81 |
|
|
| 5 |
|
| 450,000(1) |
|
| 0.81 |
|
|
| 5 |
| |
| 2,048,000 |
|
|
| 1.00 |
|
|
|
|
|
(1) Options granted vest over a period of three years |
| |||||||||
|
|
| Fiscal 2018 Activity | |
|
|
| The Company granted in the period ending August 31, 2018, 200,000 stock options with an exercise price of $0.83 and an expiration date of December 1, 2022 to an officer of the Company, pursuant to an existing management contract and stock options with an exercise price of $1.53 to directors, officers, employees and consultants that enable the option holders to purchase up to 1,725,000 common shares of the Company. | |
|
|
| A continuity schedule for stock options is presented below: |
|
| Options |
|
| Weighted Average Exercise Price $ |
|
| Weighted Average Remaining Contractual Term (Years) |
|
| Aggregate Intrinsic Value $ |
| ||||
Balance August 31, 2017 |
|
| 3,320,875 |
|
|
| 0.15 |
|
|
|
|
|
|
| ||
Exercised |
|
| (545,875 | ) |
|
| 0.17 |
|
|
|
|
|
|
| ||
Granted |
|
| 2,025,000 |
|
|
| 1.49 |
|
|
|
|
|
|
| ||
Balance August 31, 2018 |
|
| 4,800,000 |
|
|
| 0.71 |
|
|
|
|
|
|
| ||
Expired/Cancelled |
|
| (1,415,000 | ) |
|
| 0.66 |
|
|
|
|
|
|
| ||
Exercised |
|
| (430,000 | ) |
|
| 0.15 |
|
|
|
|
|
|
| ||
Granted |
|
| 2,048,000 |
|
|
| 1.00 |
|
|
|
|
|
|
| ||
Balance August 31, 2019 (Outstanding) |
|
| 5,003,000 |
|
|
| 0.89 |
|
|
| 3.34 |
|
|
| 791,800 |
|
Balance August 31, 2019 (Exercisable) |
|
| 3,961,000 |
|
|
| 0.90 |
|
|
| 3.03 |
|
|
| 752,300 |
|

| Page 63 of 83 |
| Table of Contents |
| The fair value of options granted was estimated as of the date of the grant by using the Black-Scholes option pricing model with the following assumptions: |
|
| August 31 2019 |
|
| August 31 2018 |
| ||
Expected volatility |
| 100% – 144% |
|
| 127% – 131% |
| ||
Risk-free interest rate |
| 1.42% – 2.89% |
|
| 2.13% – 2.74% |
| ||
Expected life |
| 5 years |
|
| 5 years |
| ||
Dividend yield |
| 0.00% |
| 0.00% | ||||
Estimated fair value per option |
| $0.60 - $1.07 |
|
| $0.70 – $1.73 |
| ||
15. Revenues
|
| August 31 2019 $ |
|
| August 31 2018 $ |
| ||
Product sales |
|
| 24,282 |
|
|
| 16,967 |
|
Licensing revenue (Note 11) |
|
| 198,000 |
|
|
| 415,183 |
|
Freight revenue |
|
| 328 |
|
|
| 1,137 |
|
|
|
| 222,610 |
|
|
| 433,287 |
|
| The Company recognized licensing revenue on a pro-rated basis over the term of the Licensing Agreement and additional licensing fees as they were earned. The Company has determined that the support services form an insignificant portion of the licensing contract as they are substantially completed prior to delivery of the DehydraTECH™ technology (the Technology) and that delivery of the license is complete when the Technology is transferred to the licensee. Additional licensing fees and royalties are recognized as they are earned. During the year ended August 31, 2019, the Company recognized $Nil of deferred revenue (Note 12) and $198,000 of additional Intellectual Property Licensing fees. | |
|
|
| There was a slight increase in product sales in the current year compared to the previous years as the Company was able to solve some payment processing issues later in the fiscal year. The additional Licensing fees consist of IP licensing fees for transfer of the Technology with the signing of definitive agreements for the DehydraTECH technology. The additional Licensing fees include payments due upon transfer of the Technology and installment payments that are receivable within 12 months (Note 7). | |
|
|
| 16. | Related Party Transactions |
Management, consulting and accounting services |
| Cash $ |
|
| % |
|
| Non-Cash(2) $ |
|
| % |
|
| Aug 31 2019 Total $ |
|
| Cash $ |
|
| % |
|
| Non-Cash (2) $ |
|
| % |
|
| Aug 31 2018 Total $ |
| ||||||||||
C.A.B Financial Services(1) |
|
| 223,280 |
|
|
| 100 |
|
|
| 0 |
|
|
| 0 |
|
|
| 223,280 |
|
|
| 144,000 |
|
|
| 11 |
|
|
| 1,212,269 |
|
|
| 89 |
|
|
| 1,356,269 |
|
M&E Services Ltd.(1) |
|
| 112,377 |
|
|
| 100 |
|
|
| 0 |
|
|
| 0 |
|
|
| 112,377 |
|
|
| 85,663 |
|
|
| 13 |
|
|
| 568,737 |
|
|
| 87 |
|
|
| 654,401 |
|
Docherty Management Limited(1) |
|
| 195,740 |
|
|
| 100 |
|
|
| 0 |
|
|
| 0 |
|
|
| 195,740 |
|
|
| 140,471 |
|
|
| 11 |
|
|
| 1,148,152 |
|
|
| 89 |
|
|
| 1,288,622 |
|
Company controlled by a director |
|
| 14,932 |
|
|
| 12 |
|
|
| 112,718 |
|
|
| 88 |
|
|
| 127,650 |
|
|
| 12,000 |
|
|
| 15 |
|
|
| 65,686 |
|
|
| 85 |
|
|
| 77,686 |
|
Directors |
|
| 16,138 |
|
|
| 9 |
|
|
| 172,330 |
|
|
| 91 |
|
|
| 188,468 |
|
|
| - |
|
|
| 0 |
|
|
| 65,686 |
|
|
| 100 |
|
|
| 65,686 |
|
|
|
| 562,467 |
|
|
|
|
|
|
| 285,048 |
|
|
|
|
|
|
| 847,515 |
|
|
| 382,134 |
|
|
|
|
|
|
| 3,060,530 |
|
|
|
|
|
|
| 3,442,664 |
|
| (1) C.A.B. Financial Services is owned by the CEO of the Company, M&E Services Ltd. is owned by the CFO of the Company (as of June 1 2017), and Docherty Management Limited is owned by the President of the Company. | |
| (2) Stock Based Compensation (SBC) and Share Awards are included in the total value of the grants and awards included in expenses. In the year ended August 31, 2019 the Company granted no option or awards to officers and $285,048 awards to Directors included in Consulting expense. In the year ended August 31, 2018 the Company granted a total of 1,700,000 incentive stock options to officers and directors of the Company with a fair value of $2,111,028 and included in Consulting expense (Note 14). | |

| Page 64 of 83 |
| Table of Contents |
August 31, 2018 |
| Common Shares |
|
| Fair Value |
|
| Cash |
| |||
Docherty Management (Note 13,16) (A) |
|
| 345,250 |
|
| $ | 458,305 |
|
| $ | 164,361 |
|
CAB (Note 13,16)(B) |
|
| 143,225 |
|
| $ | 192,195 |
|
| $ | 100,475 |
|
M&E Services Ltd (Note 13,16) |
|
| 41,666 |
|
| $ | 34,166 |
|
|
| - |
|
(A) Issued in lieu of issuance of 466,666 common shares, as mutually agreed to between the parties. | ||||||||||||
| (B) Issued in lieu of issuance of 216,670 common shares, as mutually agreed to between the parties. | ||||||||||||
| Due to related parties: | |
|
|
| As at August 31, 2019, $48,096 (August 31, 2018 - $7,855) was payable to related parties included in due to related parties. | |
|
|
| The related party transactions are recorded at the exchange amount established and agreed to between the related parties. | |
|
|
| 17. | Segment Information |
|
|
| The Company’s operations involve the development and usage, including licensing, of its proprietary nutrient infusion Technology. Lexaria is centrally managed and its chief operating decision makers, being the president and the CEO, use the consolidated and other financial information supplemented by revenue information by category of alternative health consumer products and technology licensing to make operational decisions and to assess the performance of the Company. The company has identified two reportable segments: Intellectual Property Licensing and Consumer Products. Licensing revenues are significantly concentrated on three licensees. |
|
| IP Licensing |
|
| Consumer Products |
|
| Corporate |
|
| Consolidated Total |
| ||||
External Revenue |
| $ | 198,000 |
|
| $ | 24,610 |
|
| $ | - |
|
| $ | 222,610 |
|
CoGS |
| $ | - |
|
| $ | (22,893 | ) |
| $ | - |
|
| $ | (22,893 | ) |
Operating Expenses |
| $ | (1,211,733 | ) |
| $ | (968,947 | ) |
| $ | (2,177,450 | ) |
| $ | (4,358,130 | ) |
Segment Loss |
| $ | (1,013,733 | ) |
| $ | (967,230 | ) |
| $ | (2,177,450 | ) |
| $ | (4,158,413 | ) |
Total Assets |
| $ | 645,969 |
|
| $ | 127,396 |
|
| $ | 1,901,854 |
|
| $ | 2,675,219 |
|
18. Commitments, Significant Contracts and Contingencies Management and Service Agreements As at August 31, 2019, the Company is party to the following contractual commitments:
Party | Monthly Commitment |
| Expiry Date | |
C.A.B Financial Services (6) |
| CAD $29,167 |
| January 1, 2022 |
Docherty Management Ltd. (6) | CAD $25,000 |
| January 1, 2022 | |
M&E Services Ltd. (1)(2) |
| CAD $12,960 |
| June 1, 2021 |
Corporate Development (3)(4) |
| CAD $1,000 |
| Month to Month |
Corporate Development (3)(4) |
| CAD $8,000 |
| Month to Month |
Investor relations and communications – Alex Blanchard Capital(1) |
| CAD $7,500 |
| Month to Month |
Office Management(7) |
| CAD $10,000 |
| August 15, 2022 |
Research & Development |
| CAD $3,854 |
| Month to Month |
Office Rent(5) |
| CAD $4,823 |
| November 15, 2023 |
| Revenue Incentive Milestones | |
| (1) | 100,000 common shares issuable upon the Company achieving non-refundable revenues of $200,000 to any single customer in any consecutive 60-day period for the first 12 months of the contract, plus a further 50,000 common shares issuable upon achieving non-refundable revenues of $200,000 to any single customer in any consecutive 60-day period, during the 13th - 24th months of the contract. If the Company achieves non-refundable revenues of $500,000 in any fiscal quarter, a further 200,000 common shares may be issuable during the first 12 months of the contract and 100,000 common shares during the 13th - 24th months of the contract. |

| Page 65 of 83 |
| Table of Contents |
| Intellectual Property Milestones | |
| (2) | During the term of the agreement, for each provisional patent application substantively devised and successfully created, written, and filed with the U.S. Patent Office for the Company’s Technology, 250,000 restricted common shares of the Company will be issuable. |
|
|
|
| Corporate Development Milestones | |
| (3) | For new customers sourced by a Consultant for the first 12 months of the contract; for combined Lexaria Energy and ViPova products and including all combined sales efforts and/or technology licensing revenues, achieving non-refundable revenues of $200,000 to any single customer in any consecutive 60-day period would result in a restricted common share award of 100,000 Company shares (not achieved); and, during the 13th - 24th months of the contract; a restricted common share award of 50,000 Company shares may be achieved; this clause is limited to one payment per customer during the 12-month period, but payable on each customer that meets these sales/licensing thresholds. |
|
|
|
| (4) | For new customers sourced by a Consultant for the first 12 months of the contract; for combined Lexaria Energy and ViPova products and including all combined sales efforts and/or technology licensing revenues, achieving non-refundable revenues of $500,000 in any fiscal quarter would result in a restricted common share award of 200,000 Company shares (not achieved); and, during the 13th - 24th months of the contract; for combined Lexaria Energy and ViPova products and including all sales efforts, achieving non-refundable revenues of $500,000 in any fiscal quarter would result in a restricted common share award of 100,000 Company shares; this clause is limited to one payment per fiscal quarter. |
|
|
|
| Corporate Offices | |
| (5) | Corporate office and R&D lab space leased in Kelowna, British Columbia, Canada until November 15, 2023 with an option to extend an additional five years. Base rent is CDN$12.56 per square foot until November 14, 2019, CDN$12.86 per square foot until November 14, 2021 and CDN$13.21 per square foot until November 14, 2023 plus common area maintenance and taxes. |
|
|
|
| Performance Incentives | |
| (6) | A performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the board of directors of Lexaria. Compensation equal to 2% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. Certain compensation to be paid upon a change of control excluding certain circumstances and participation in the Company’s approved stock option plans. |
|
|
|
| (7) | Compensation equal to 0.4% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. Certain compensation to be paid upon a change of control excluding certain circumstances and participation in the Company’s approved stock option plans. |
19. Prepaid Expenses Prepaid expenses consist of the following at August 31, 2019 and August 31, 2018:
|
| August 31 2019 $ |
|
| August 31 2018 $ |
| ||
Advertising & Conferences |
|
| 39,143 |
|
|
| 137,654 |
|
Consulting Fees |
|
| - |
|
|
| 4,555 |
|
Office & Insurance |
|
| 29,784 |
|
|
| 21,533 |
|
Legal Fees |
|
| - |
|
|
| 29,990 |
|
|
|
| 68,927 |
|
|
| 193,732 |
|

| Page 66 of 83 |
| Table of Contents |
20. Income Tax The following table reconciles the income tax benefit at the U.S. Federal statutory rate to income tax benefit at the Company’s effective tax rates as at August 31, 2019 and 2018:
|
| August 31 2019 $ |
| August 31 2018 $ | ||||
| ||||||||
Loss before taxes |
| (4,158,413 | ) |
| (6,609,187 | ) | ||
Expected income tax recovery |
| (883,841 | ) |
| (1,322,068 | ) | ||
Non-deductible items |
| 8,544 |
| 2,724 | ||||
Change in estimates |
| 948 |
| (54,057) |
| |||
Effect of changes in foreign and long-term tax rates |
| - |
| 1,816,659 |
| |||
Change in valuation allowance |
| 874,349 |
| (443,258 | ) | |||
Total income taxes |
| - |
| - | ||||
Deferred taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes. Deferred tax assets at August 31, 2019 and 2018 are comprised of the following: |
|
| August 31 2019 $ |
|
| August 31 2018 $ |
| ||
Non-capital losses |
|
| 5,022,441 |
|
|
| 4,130,915 |
|
Marketable securities |
|
| 2,300 |
|
|
| 1,789 |
|
Property plant and equipment |
|
| - |
|
| 24 |
| |
Total unrecognized deferred tax assets |
|
| 5,024,741 |
|
|
| 4,132,728 |
|
The Company has net operating loss carry-forwards of approximately $24,457,000 which may be carried forward to apply against future year income tax for U.S. tax purposes. |
Year |
| Amount |
| |
2025 |
|
| 76,000 |
|
2026 |
|
| 508,000 |
|
2027 |
|
| 1,056,000 |
|
2028 |
|
| 720,000 |
|
2029 |
|
| 753,000 |
|
2030 |
|
| 552,000 |
|
2031 |
|
| 538,000 |
|
2032 |
|
| 252,000 |
|
2033 |
|
| 344,000 |
|
2034 |
|
| 3,257,000 |
|
2035 |
|
| 1,934,000 |
|
2036 |
|
| 1,150,000 |
|
2037 |
|
| 1,857,000 |
|
Indefinite |
|
| 11,249,000 |
|
|
|
| 24,246,000 |
|

| Page 67 of 83 |
| Table of Contents |
21. Marketable Securities The components of Marketable Securities were as follows:
|
| Cost Basis $ |
|
| Unrealized Gains $ |
|
| Unrealized Losses $ |
|
| Total $ |
| ||||
August 31, 2018 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Common Stock |
|
| 25,000 |
|
|
| - |
|
|
| (14,849 | ) |
|
|
| |
Total |
|
| 25,000 |
|
|
| - |
|
|
| (14,849 | ) |
|
| 10,151 |
|
August 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
| 81,250 |
|
|
| 9,335 |
|
|
| (12,124 | ) |
|
|
| |
Total |
|
| 81,250 |
|
|
| 9,335 |
|
|
| (26,973 | ) |
|
| 63,612 |
|
| Unrealized losses from common stock are due to market price movements. Management does not believe any remaining unrealized losses represent other-than-temporary impairments based on our evaluation of available evidence. |
22. | Subsequent Events |
|
|
| November 5, 2019 the Company pursuant to certain consulting agreements issued a total of 1,000,000 stock options at $0.55 per Share for a period of five years; and 225,000 warrants at $0.80 for a period of three years. |
|
|
| November 13, 2019 Lexaria closed the first tranche of its previously announced private placement. 1,554,245 units were issued at $0.45 for a total of $699,410.25. Each unit consists of one common share and one warrant exercisable at $0.80 until November 13, 2020, thereafter at $1.20 until November 13, 2021. The Company paid $3,937.50 and issued 8,750 broker warrants. |

| Page 68 of 83 |
| Table of Contents |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
There were no disagreements related to accounting principles or practices, financial statement disclosure, internal controls or auditing scope or procedure during the two fiscal years and their respective interim periods.
Item 9A. Controls and Procedures
Management’s Report on Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms, and that such information is accumulated and communicated to our management, including our president and chief executive officer (also our principal executive officer) and our chief financial officer (also our principal financial and accounting officer) to allow for timely decisions regarding required disclosure.
As of August 31, 2019, the end of our fiscal year covered by this report, we carried out an evaluation, under the supervision and with the participation of our President and chief executive officer and chief financial officer (also our principal executive and financial reporting and accounting officers), of the effectiveness of the design and operation of our disclosure controls and procedures. Based on the foregoing, our president, chief executive officer and the chief financial officer concluded that our disclosure controls and procedures were not effective, because of material weaknesses in our internal control over financial reporting, as of the end of the period covered by this annual report, as discussed below.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Responsibility, estimates and judgments by management are required to assess the expected benefits and related costs of control procedures. The objectives of internal control include providing management with reasonable, but not absolute, assurance that assets are safeguarded against loss from unauthorized use or disposition, and that transactions are executed in accordance with management’s authorization and recorded properly to permit the preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States. Our management assessed the effectiveness of our internal control over financial reporting as of August 31, 2019. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework. Our management has concluded that, as of August 31, 2019, our internal control over financial reporting is not effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with US generally accepted accounting principles due to the existence of material weaknesses. Our management reviewed the results of their assessment with our Board of Directors.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statement will not be prevented or detected on a timely basis. Our management identified the following material weaknesses:
We did not complete documented internal control testing and did not document detailed risk assessment and sampling methodology.
We lack a specific written policy and procedure when dealing with a whistleblower complaint.
We lack detailed controls regarding the understanding and application of ASC 310 with respect to our treatment of “incurred credit losses”.
To address these material weaknesses, management performed additional analyses and other procedures to ensure that the financial statements included herein fairly present, in all material respects, our financial position, results of operations and cash flows for the periods presented. Accordingly, we believe that the financial statements included in this report fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented.

| Page 69 of 83 |
| Table of Contents |
Remediation
In response to these material weaknesses discussed above, we intend to: (1) increase our accounting personnel when funds are available which will also permit better segregation of duties; (2) we have appointed (August 15, 2019) an additional independent director to our audit committee; (3) Establish a formal Whistleblower policy and procedural document for our employees and consultants; and (4) review and update our controls and processes regarding ASC 310.
We will continue to monitor and evaluate the effectiveness of our internal controls and procedures over financial reporting on an ongoing basis and are committed to taking further action and implementing additional improvements as necessary and as funds allow.
This annual report includes an adverse attestation report of our company’s registered public accounting firm regarding internal control over financial reporting.
Inherent limitations on Effectiveness of Controls
Internal control over financial reporting has inherent limitations which include but is not limited to the use of independent professionals for advice and guidance, interpretation of existing and/or changing rules and principles, segregation of management duties, scale of organization, and personnel factors. Internal control over financial reporting is a process which involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements on a timely basis, however these inherent limitations are known features of the financial reporting process and it is possible to design into the process safeguards to reduce, though not eliminate, this risk. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
During the year ended August 31, 2019 our controls and controls processes during the period were updated and revised based on the additional personnel added to our company, their functions and regulatory requirements from Health Canada. The fundamental control processes remained consistent with prior years but were expanded for new operational areas, additional subsidiaries and enhanced through additional resources. There have been no changes in our internal controls over financial reporting that occurred during the year ended August 31, 2019 that have materially or are reasonably likely to materially affect, our internal controls over financial reporting.
None.

| Page 70 of 83 |
| Table of Contents |
PART III
Item 10. Directors, Executive Officers and Corporate Governance
All directors of our company hold office until the next annual meeting of the security holders or until their successors have been elected and qualified. The officers of our company are appointed by our board of directors and hold office until their death, resignation or removal from office. Our directors and executive officers, their ages, positions held, and duration as such, are as follows:
Name |
| Position Held with our Company |
| Age |
| Date First Elected Or Appointed |
| Date of Resignation |
Christopher Bunka |
| Chairman, Chief Executive Officer, and Director |
| 58 |
| October 26, 2006 |
| - |
John Docherty |
| President and Director |
| 49 |
| April 15, 2015 April 29, 2016 |
| - |
Allan Spissinger |
| Chief Financial Officer |
| 50 |
| June 1, 2017 |
| - |
Nicholas Baxter |
| Director |
| 66 |
| July 8, 2011 |
| - |
Ted McKechnie |
| Director |
| 72 |
| September 16, 2015 |
| - |
Brian Quigley |
| Director |
| 46 |
| August 14, 2019 |
| - |
Business Experience
The following is a brief account of the education and business experience of each director and executive officer during the past five years, indicating each person’s principal occupation during the period, and the name and principal business of the organization by which he was employed.
Mr. Christopher Bunka – Chairman, Chief Executive Officer and Director
Mr. Bunka has been Chairman of the Board and CEO since 2006 and was primarily responsible for the corporate pivot from older business activities to bioscience. Mr. Bunka is a serial entrepreneur and has been involved in several private and public companies since the late 1980’s. He was well known for more than a decade as a part-time business commentator in print and radio, as well as an author. He has extensive experience in the capital markets, corporate governance, project acquisition and corporate finance. He is a named inventor on some of Lexaria’s pending patents.
Since 1988, Mr. Bunka has been the CEO of CAB Financial Services Ltd., a private holding company located in Kelowna, Canada. He is a venture capitalist and corporate consultant.
Mr. John Docherty – President and Director
Mr. Docherty was appointed President of Lexaria effective April 15, 2015. Prior to Lexaria Mr. Docherty was former President and Chief Operating officer of Helix BioPharma Corp. (TSX: HBP), where he led the company’s pharmaceutical development programs for its plant and recombinantly derived therapeutic protein product candidates.
Mr. Docherty is a senior operations and management executive with over 20 years experience in the pharmaceutical and biopharmaceutical sectors. He has worked with large multinational companies and emerging, private and publicly held start-ups. At Helix, Mr. Docherty was also instrumental in the areas of investor/stakeholder relations, capital raising, capital markets development, strategic partnering, regulatory authority interactions and media relations, and he also served as a management member of its board of directors. Prior to this, Mr. Docherty was President and a board member of PharmaDerm Laboratories Ltd., a Canadian drug delivery company that developed unique microencapsulation formulation technologies for use with a range of active compounds.
Mr. Docherty has also held positions with companies such as Astra Pharma Inc., Nu-Pharm Inc. and PriceWaterhouseCoopers’ former global pharmaceutical industry consulting practice. He is a named inventor on issued and pending patents and he has a M.Sc. in pharmacology and a B.Sc. in Toxicology from the University of Toronto.
He has served as a director of Lexaria since April 29, 2016.
Mr. Allan Spissinger – Chief Financial Officer
Prior to concentrating on finance and accounting, Mr. Spissinger worked within the Informational Technologies (IT) sector for over a decade; specializing in corporate IT infrastructure and software development projects. Mr. Spissinger joined the audit and assurance department at PricewaterhouseCoopers (PwC) where he obtained his Chartered Professional Accountant (CPA) designation focusing on financial reporting and Sarbanes-Oxley (SOX) compliance in the following sectors: resources, manufacturing and technologies. Mr. Spissinger joined Lexaria in September 2014 as a corporate controller. His positive mentorship, excellent communication and extensive leadership skills have enabled him to successfully manage a variety of private businesses for over 20 years.

| Page 71 of 83 |
| Table of Contents |
Mr. Nicholas Baxter - Director
Mr. Baxter was appointed as a member on the board of directors of Lexaria Corp. in 2009. Mr. Baxter received a Bachelor of Science (Honours) from the University of Liverpool in 1975, and has worked on oil & gas projects in many areas of the world. Since the 1980’s, he has worked with companies in the public markets both in the U.K. and in Canada. Mr. Baxter brings extensive real-world experience as a board member.
Mr. Ted McKechnie – Director
Mr. McKechnie is a well-recognized thought leader in the Canadian food industry. In the past, Mr. McKechnie was president of Maple Leaf Foods, an owner and senior executive at Humpty Dumpty and a senior leader at Pepsi Co. After a distinguished career as an executive and marketer specializing in food manufacturing, he now focuses on moving the Canadian food sector into the future. Besides being the chairman of Food Starter’s board, Mr. McKechnie is also the Chairman/CEO of The Davies Group and William Davies Consulting Inc. Mr. McKechnie is also a chairman of the board for Advanced Technology For Food Manufacturing, and the Director of Lexaria Bioscience Corporation.
Mr. McKechnie is often called upon by think tanks, the government and industry leaders to offer insights on how to grow the food sector and add more value to the Canadian economy.
Mr. Brian Quigley - Director
Mr. Quigley has been a senior Consumer Packaged Goods executive for over 20 years of Brand Building, Marketing, Operations, Leadership and General Management experience leading business transformations that deliver shareholder returns for public and private equity investors. Mr. Quigley is one of the founders of Green Sky Strategy. Before founding Green Sky, he spent 16 years at the Altria Group, with 7 years as President & CEO for U.S. Smokeless Tobacco and Nu-Mark, Altria’s innovation Company. In his time at Altria, Brian spearheaded the companies Harm Reduction strategies and worked to deliver results by creating change in the U.S. Tobacco business. Prior to Altria, Brian held branding and leadership roles with several companies, including Pinnacle Foods Corporation, International Home Foods, which is now part of ConAgra, Inc., and in the advertising industry. Brian has launched dozens of new products, created consumer focused innovation strategies and built businesses and cultures that deliver results. Brian is motivated by helping to change lives with meaningful brands.
Family Relationships
There are no family relationships among any of our directors or officers.
Involvement in Certain Legal Proceedings
None of our directors, executive officers, promoters or control persons has been involved in any of the following events during the past five years:
| a. | A petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing; |
| 2) | Such person was convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses); |
|
|
|
| 3) | Such person was the subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities: |
| i. | Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity |
|
|
|
| ii. | Engaging in any type of business practice; or |
|
|
|
| iii. | Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws; |

| Page 72 of 83 |
| Table of Contents |
| 4) | Such person was the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in paragraph (f)(3)(i) of this section, or to be associated with persons engaged in any such activity; |
|
|
|
| 5) | Such person was found by a court of competent jurisdiction in a civil action or by the Commission to have violated any Federal or State securities law, and the judgment in such civil action or finding by the Commission has not been subsequently reversed, suspended, or vacated; |
|
|
|
| 6) | Such person was found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated; |
|
|
|
| 7) | Such person was the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of: |
| i. | Any Federal or State securities or commodities law or regulation; or |
|
|
|
| ii. | Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or |
|
|
|
| iii. | Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| 8) | Such person was the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Delinquent Section 16(a) Reports
Section 16(a) of the Securities Exchange Act of 1934 requires our executive officers and directors and persons who own more than 10% of our common stock to file with the Securities and Exchange Commission initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our common stock and other equity securities, on Forms 3, 4 and 5 respectively. Executive officers, directors and greater than 10% shareholders are required by the SEC regulations to furnish us with copies of all Section 16(a) reports that they file.
Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that during fiscal year ended August 31, 2019, all filing requirements applicable to our officers, directors and greater than 10% percent beneficial owners were complied with.
Code of Ethics
We adopted a Code of Ethics applicable to our senior financial officers and certain other finance executives, which is a “code of ethics” as defined by applicable rules of the SEC. Our Code of Ethics is attached as an exhibit to our Form SB-2 filed on September 20, 2007. If we make any amendments to our Code of Ethics other than technical, administrative, or other non-substantive amendments, or grant any waivers, including implicit waivers, from a provision of our Code of Ethics to our chief executive officer, chief financial officer, or certain other finance executives, we will disclose the nature of the amendment or waiver, its effective date and to whom it applies in a Current Report on Form 8-K filed with the SEC.
Board and Committee Meetings
Our board of directors held four formal meetings and several informal meetings during the year ended August 31, 2019. All proceedings of the board of directors were conducted by resolutions consented to in writing by all the directors and filed with the minutes of the proceedings of the directors. Such resolutions consented to in writing by the directors entitled to vote on that resolution at a meeting of the directors are, according to the Nevada General Corporate Law and our Bylaws, as valid and effective as if they had been passed at a meeting of the directors duly called and held.

| Page 73 of 83 |
| Table of Contents |
Nomination Process
As of August 31, 2019, we did not effect any material changes to the procedures by which our shareholders may recommend nominees to our board of directors. Our board of directors does not have a policy with regards to the consideration of any director candidates recommended by our shareholders. Our board of directors has determined that it is in the best position to evaluate our company’s requirements as well as the qualifications of each candidate when the board considers a nominee for a position on our board of directors. If shareholders wish to recommend candidates directly to our board, they may do so by sending communications to the president of our Company at the address on the cover of this annual report.
Audit Committee and Audit Committee Financial Expert
Currently our audit committee consists of Chris Bunka, Ted McKechnie and Nicholas Baxter. We currently do not have nominating, compensation committees or committees performing similar functions. There has not been any defined policy or procedure requirements for shareholders to submit recommendations or nomination for directors.
Our board of directors has determined that it does not have a member of its board of directors (audit committee) that qualifies as an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K, and is “independent” as the term is used in Item 7(d)(3)(iv) of Schedule 14A under the Securities Exchange Act of 1934, as amended.
We believe that the members of our board of directors are collectively capable of analyzing and evaluating our consolidated financial statements and understanding internal controls and procedures for financial reporting. We believe that retaining an independent director who would qualify as an “audit committee financial expert” would be overly costly and burdensome and is not warranted in our circumstances given the early stages of our development and the fact that we have not generated any material revenues to date. In addition, we currently do not have nominating, compensation or audit committees or committees performing similar functions nor do we have a written nominating, compensation or audit committee charter. Our board of directors does not believe that it is necessary to have such committees because it believes the functions of such committees can be adequately performed by our board of directors.
Item 11. Executive Compensation
The particulars of the compensation paid to the following persons:
| a) | our principal executive officer; |
|
|
|
| b) | each of our two most highly compensated executive officers who were serving as executive officers at the end of the years ended August 31, 2019 and August 31, 2018; and |
|
|
|
| c) | up to two additional individuals for whom disclosure would have been provided under (b) but for the fact that the individual was not serving as our executive officer at the end of the years ended August 31, 2019 and August 31, 2018, |
who we will collectively refer to as the named executive officers of our Company, are set out in the following summary compensation table, except that no disclosure is provided for any named executive officer, other than our principal executive officers, whose total compensation did not exceed $100,000 for the respective fiscal year:

| Page 74 of 83 |
| Table of Contents |
SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||||||||||||
Name and Principal Position |
| Year |
|
| Salary ($) |
|
| Bonus ($) |
|
| Stock Awards($) |
|
| Option Awards ($) |
|
| Non-Equity Incentive Plan Compensation($) |
|
| Non-Qualified Deferred Compensation Earnings ($) |
|
| All Other Compensation($) |
|
| Total ($) |
| |||||||||
Christopher Bunka(1), |
|
| 2019 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 223,280 |
|
|
| 223,280 |
|
Chairman, Chief Executive |
| 2018 |
|
|
| - |
|
|
| - |
|
|
| 292,669 | (4) |
|
| 919,600 | (6) |
|
| - |
|
|
| - |
|
|
| 144,000 |
|
|
| 1,356,269 |
| |
Officer & Director |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
John Docherty(2) |
| 2019 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 195,740 |
|
|
| 195,740 |
| |
President & Director |
| 2018 |
|
|
| - |
|
|
| - |
|
|
| 622,666 | (3) |
|
| 525,486 | (6) |
|
| - |
|
|
| - |
|
|
| 140,471 |
|
|
| 1,288,623 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allan Spissinger(8) |
|
| 2019 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 112,377 |
|
|
| 112,37765 |
|
Chief Financial Officer |
| 2018 |
|
|
| - |
|
|
| - |
|
|
| 34,166 | (5) |
|
| 534,571(6) | (7) |
|
| - |
|
|
| - |
|
|
| 85,663 |
|
|
| 654,400 |
| |
| (1) | Mr. Bunka was appointed as chairman, president, chief executive officer, and director on October 26, 2006, and was chief financial officer of our company from April 29, 2016 to May 31 2017. He resigned as president on April 15, 2015. We pay Mr. Bunka a consulting fee through CAB Financial Services Ltd., where he is also the Chief Executive Officer. |
| (2) | Mr. Docherty became President on April 15, 2015 and a director on April 29, 2016. We pay Mr. Docherty a consulting fee through his wholly owned company Docherty Management Ltd. |
| (3) | Pursuant to the agreement with Docherty Management Ltd. Mr. Docherty received 466,666 (2017 - 462,000) common shares with a value of $622,666 (2017 - $97,710). |
| (4) | Pursuant to the agreement with CAB Financial Services Ltd. Mr. Bunka received 216,670 (2016 - 210,000) common shares with a value of $292,670 (2017 - $61,950). |
| (5) | Mr. Spissinger became Interim Chief Financial Officer on June 1, 2017 and Chief Financial Officer June 1, 2018. We pay Mr. Spissinger a consulting fee through his wholly owned company M&E Services Ltd. |
| (6) | The fair value of the stock options awarded was estimated using the Black-Scholes option pricing model with the following assumptions: expected volatility of 130%; risk-free interest rate of 2.68%; expected life of 5 years; and dividend yield of 0%. |
| (7) | The fair value of the stock options awarded was estimated using the Black-Scholes option pricing model with the following assumptions: expected volatility of 129%; risk-free interest rate of 2.13%; expected life of 5 years; and dividend yield of 0%. |
Our company is currently paying consulting fees to our Chief Executive Officer CAD$29,167 per month, our President CAD$25,000 per month and our Chief Financial Officer CAD$12,960 per month.
Consulting Agreements
The Company had an agreement with CAB for a consulting fee of $144,000 per year and has negotiated a 3-year term renewal management contract with Chief Executive Officer Chris Bunka retroactively effective January 1, 2019. The annual compensation payable is CDN$350,000 per year and the following performance incentives.
| · | Performance Incentives |
A performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the board of directors of Lexaria. Compensation equal to 2% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. Certain compensation to be paid upon a change of control excluding certain circumstances and participation in the Company’s approved stock option plans.

| Page 75 of 83 |
| Table of Contents |
The Company appointed Mr. John Docherty as President of Lexaria effective April 15, 2015. The Company had an agreement with Docherty Management Limited, solely owned by Mr. John Docherty with compensation of CAD$180,000 plus applicable taxes per year and has negotiated a 3-year term renewal management contract CAD$300,000 per year and the following performance incentives.
| · | Performance Incentives as defined above. |
On June 1, 2018, the Company executed a thirty six month contract with M&E Services Ltd., a wholly owned company by Mr. Allan Spissinger, as Chief Financial Officer with monthly compensation of CAD$12,000 plus applicable taxes, including an annual 8% increase, superseding the previous agreement for $8,000 per month plus applicable taxes.
Other than as set out in this annual report on Form 10-K we have not entered into any employment or consulting agreements with any of our current officers, directors or employees.
Grants of Plan-Based Awards Table
We did not grant any awards to our named executive officers in the during our fiscal year ended August 31, 2019.
Outstanding Equity Awards at Fiscal Year End
The particulars of unexercised options, stock that has not vested and equity incentive plan awards for our named executive officers are set out in the following table:
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | ||||||||||
| OPTION AWARDS | STOCK AWARDS | ||||||||
Name | Number of Securities Underlying Unexercised Options Exercisable (#) | Number of Securities Underlying Unexercised Options Unexercisable (#) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) | |
Christopher Bunka | 700,000 | - | - | $1.53 | 2023/05/31 | - | - | - | - | |
John Docherty | 550,000 300,000 400,000 | - - - | - - - | $0.10 $0.11 $1.53 | 2020/03/26 2021/04/15 2023/05/31 | - - - | - - - | - - - | - - - | |
Allan Spissinger | 150,000 200,000 300,000 | - - - | - - - | $0.37 $0.83 $1.53 | 2022/06/01 2022/11/30 2023/05/31 | - - - | - - - | - - - | - - - | |
Option Exercises
During our fiscal year ended August 31, 2019, Mr. Allan Spissinger exercised 50,000 options previously granted at $0.37.
Compensation of Directors
As of January 2019, we implemented agreements for compensating our directors for their services in their capacity as directors for CAD$30,000 per year paid quarterly in advance. As of August 31, 2019, three of our Directors are accepting compensation for their services.

| Page 76 of 83 |
| Table of Contents |
Pension, Retirement or Similar Benefit Plans
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We have no material bonus or profit sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that stock options may be granted at the discretion of the board of directors or a committee thereof.
Indebtedness of Directors, Senior Officers, Executive Officers and Other Management
None of our directors or executive officers or any associate or affiliate of our company during the last two fiscal years is or has been indebted to our company by way of guarantee, support agreement, letter of credit or other similar agreement or understanding currently outstanding.
Compensation Committee Interlocks and Insider Participation
During 2019, we did not have a compensation committee or another committee of the board of directors performing equivalent functions. Instead the entire board of directors performed the function of compensation committee. Our board of directors approved the executive and director compensation updates with the entire board acting as the compensation committee. Updated compensation is as disclosed in this Form 10-K.
Compensation Committee Report
None.
The following table sets forth, as of October 21, 2019, certain information with respect to the beneficial ownership of our common shares by each shareholder known by us to be the beneficial owner of more than 5% of our common shares, as well as by each of our current directors and executive officers as a group. Each person has sole voting and investment power with respect to the shares of common stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of common stock, except as otherwise indicated.
Name and Address of Beneficial Owner |
| Amount and Beneficial Ownership |
|
| Percentage of Class |
| ||
Christopher Bunka; Kelowna BC Canada |
|
| 13,908,148(1) |
|
| 17.65% | ||
Nicholas Baxter; Aberdeenshire, UK* |
|
| 480,000(2) |
|
| 0.61% | ||
John Docherty; Toronto, Ontario |
|
| 2,872,250(3) |
|
| 3.65% | ||
Ted McKechnie; Toronto, Ontario* |
|
| 545,738(4) |
|
| 0.69% | ||
Allan Spissinger; Langley, BC* |
|
| 769,166(5) |
|
| 0.98% | ||
Brian Quigley; Richmond, VA* |
|
| 100,000(6) |
|
| 0.13% | ||
Directors and Executive Officers as a Group (6 persons) |
|
| 18,675,302 |
|
|
| 23.70% | |
| * Less than 1% | |
(1) Includes 6,081,844 shares held in the name of C.A.B. Financial Services and 7,126,304 shares held directly by Chris Bunka, chairman, chief executive officer and a director of our Company. Includes 700,000 options which are exercisable at $1.53. (2) Includes 110,000 options which are exercisable at $0.10 and 150,000 options exercisable at $0.99. Nicholas Baxter is a director of our Company. (3) Includes 550,000 options which are exercisable at $0.10, 300,000 options which are exercisable at $0.11, and 400,000 options exercisable at $1.53. John Docherty is the President and a Director of our Company (4) Includes 110,000 options exercisable at $0.17 and 150,000 exercisable at $0.99. Ted McKechnie is a Director of our Company. (5) Includes 150,000 options exercisable at $0.37, 200,000 exercisable at $0.83 and 300,000 exercisable at $1.53. Allan Spissinger is chief financial officer of our Company. (6) Includes 100,000 options exercisable at $0.81. Brian Quigley is a Director of our Company. (7) Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person’s actual ownership or voting power with respect to the number of shares of common stock actually outstanding on November 5, 2019. As of November 5, 2019, there were 78,787,134 shares of our common stock issued and outstanding. |

| Page 77 of 83 |
| Table of Contents |
Changes in Control
We are unaware of any contract or other arrangement the operation of which may at a subsequent date result in a change in control of our company.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Except as disclosed herein, no director, executive officer, shareholder holding at least 5% of shares of our common stock, or any family member thereof, had any material interest, direct or indirect, in any transaction, or proposed transaction since the year ended August 31, 2019, in which the amount involved in the transaction exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at the yearend for the last three completed fiscal years.
Director Independence
We currently act with five directors, consisting of Christopher Bunka, John Docherty, Nicholas Baxter, Brian Quigley and Ted McKechnie. We have determined that Nicholas Baxter, Ted McKechnie and Brian Quigley are “independent directors” as defined in NASDAQ Marketplace Rule 4200(a)(15).
Currently our audit committee consists of our Chris Bunka, Ted McKechnie, Brian Quigley and Nicholas Baxter. We currently do not have nominating, compensation committees or committees performing similar functions. There has not been any defined policy or procedure requirements for shareholders to submit recommendations or nomination for directors.
Our board of directors has determined that it does not have a member of its audit committee who qualifies as an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K.
From inception to present date, we believe that the members of our audit committee and the board of directors have been and are collectively capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting.
We do not have a standing compensation or nominating committee, but our entire board of directors act in such capacity. We believe that our directors are capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting. Our directors do not believe that it is necessary to have an audit committee because we believe that the functions of an audit committee can be adequately performed by the board of directors. In addition, we believe that retaining additional independent directors who would qualify as an “audit committee financial expert” would be overly costly and burdensome and is not warranted in our circumstances given the early stages of our development.
Item 14. Principal Accounting Fees and Services
The aggregate fees billed for the most recently completed fiscal year ended August 31, 2019 and for fiscal year ended August 31, 2018 for professional services rendered by the principal accountant for the audit of our annual financial statements and review of the financial statements included in our quarterly reports on Form 10-Q and services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for these fiscal periods were as follows:
|
| Year Ended |
| |||||
|
| August 31, |
|
| August 31, |
| ||
Audit Fees |
|
| 61,787 |
|
|
| 39,972 |
|
Audit Related Fees |
|
| - |
|
|
| - |
|
Tax Fees |
|
| - |
|
|
| - |
|
All Other Fees |
|
| - |
|
|
| - |
|
Total |
|
| 61,787 |
|
|
| 39,972 |
|
Audit Fees: Audit fees consist of fees billed for professional services rendered for the audits of our financial statements, reviews of our interim financial statements included in quarterly reports, services performed in connection with filings with the Securities and Exchange Commission and related comfort letters and other services that are provided by the Company’s principal accountants for the fiscal years ended August 31, 2019 and August 31, 2018 in connection with statutory and regulatory filings or engagements.

| Page 78 of 83 |
| Table of Contents |
Audit related Fees: Audit related fees consist of fees billed for assurance and related services by the Company’s principal accountant that are reasonably related to the performance of the audit or review of the Company’s financial statements, which are not included in the Audit Fees described above.
Tax Fees: Tax fees consist of fees billed for professional services for tax compliance, tax advice and tax planning. These services include assistance regarding federal, state and local tax compliance and consultation in connection with various transactions and acquisitions.
We do not use our principal accountants for services other than the ones related to the our annual audit and the review of our interim financial statements. We therefore do not involve our principal accountants for matters related to tax compliance and financial information system design and implementation. These services, which include corporate tax preparation and designing or implementing a system that aggregates source data underlying the financial statements or generates information that is significant to our financial statements, are provided internally or by other service providers.
Effective May 6, 2003, the Securities and Exchange Commission adopted rules that require that before our independent auditors are engaged by us to render any auditing or permitted non-audit related service, the engagement be:
| · | approved by our audit committee (which consists of our entire board of directors); or |
|
|
|
| · | entered into pursuant to pre-approval policies and procedures established by the board of directors, provided the policies and procedures are detailed as to the particular service, the board of directors is informed of each service, and such policies and procedures do not include delegation of the board of directors’ responsibilities to management. |
Our board of directors pre-approves all services provided by our independent auditors. All of the above services and fees were reviewed and approved by the board of directors either before or after the respective services were rendered.
Our board of directors has considered the nature and amount of fees billed by our independent auditors and believes that the provision of services for activities unrelated to the audit is compatible with maintaining our independent auditors’ independence.

| Page 79 of 83 |
| Table of Contents |
PART IV
Item 15. Exhibits, Financial Statement Schedules
a) Financial Statements
| 1) | Financial statements for our Company are listed in the index under Item 8 of this document |
|
|
|
| 2) | All financial statement schedules are omitted because they are not applicable, not material or the required information is shown in the financial statements or notes thereto. |
b) Exhibits
Exhibit Number |
| Description |
(2) |
| Plan of Acquisition, Reorganization, Arrangement, Liquidation or Succession |
| Plan of Conversion (included as Schedule “A” to the proxy statement/prospectus) | |
|
| |
(3)* |
| Articles of Incorporation and Bylaws |
| ||
| ||
|
| |
(4) |
| Instruments Defining the Rights of Security Holders, including Indentures |
| ||
| ||
| ||
| ||
| ||
|
| |
(5) |
| Opinion regarding Legality |
| Opinion of Macdonald Tuskey regarding the legality of the securities being registered | |
|
| |
(8) |
| Opinions regarding Tax Matters |
| Opinion of Dale Matheson Carr-Hilton Labonte LLP regarding U.S. tax matters | |
| Opinion of Dale Matheson Carr-Hilton Labonte LLP regarding Canadian tax matters |

| Page 80 of 83 |
| Table of Contents |

| Page 81 of 83 |
| Table of Contents |
(21) |
| Subsidiaries |
21.1 |
| Lexaria Canpharm ULC, a British Columbia Canada corporation |
21.2 |
| Poviva Corp, a Nevada corporation |
21.3 |
| Lexaria Hemp Corp., a Delaware corporation |
21.4 |
| Lexaria Nicotine LLC, a Delaware corporation |
21.5 |
| Lexaria Canpharm Holding Corp., a Nevada corporation |
21.6 |
| Lexaria Pharma Corp., a Delaware corporation |
|
| |
(23) |
| Consents of Experts and Counsel |
| ||
| Consent of Dale Matheson Carr-Hilton Labonte LLP (Included in Exhibit 8.1) | |
| Consent of Dale Matheson Carr-Hilton Labonte LLP (Included in Exhibit 8.2) | |
| Consent of Davidson & Company LLP, Chartered Professional Accountants | |
| ||
| ||
|
| |
(31) |
| Rule 13(a) - 14 (a)/15(d) - 14(a) |
| Section 302 Certifications under Sarbanes-Oxley Act of 2002 of Principal Executive Officer | |
| ||
(32) |
| Section 1350 Certifications |
| Section 906 Certification under Sarbanes Oxley Act of 2002 of Principal Executive Officer | |
| ||
|
| |
(101)** |
| Interactive Data Files |
101.INS |
| XBRL Instance Document |
101.SCH |
| XBRL Taxonomy Extension Schema Document |
101.CAL |
| XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
| XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
| XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
| XBRL Taxonomy Extension Presentation Linkbase Document |
*Incorporated by reference to same exhibit filed with the Company’s Registration Statement on Form SB-2 dated January 10, 2006.
** Furnished herewith. Pursuant to Rule 406T of Regulation S-T, the Interactive Data Files on Exhibit 101 hereto are deemed not filed or part of any registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, are deemed not filed for purposes of Section 18 of the Securities and Exchange Act of 1934, and otherwise are not subject to liability under those sections.

| Page 82 of 83 |
| Table of Contents |
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
LEXARIA BIOSCIENCE CORP.
By: /s/ Christopher Bunka
Christopher Bunka
Chief Executive Officer, Chairman and Director
(Principal Executive Officer)
Date: November 14, 2019
In accordance with the Exchange Act, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
By: /s/ Christopher Bunka
Christopher Bunka
Chief Executive Officer, Chairman and Director
(Principal Executive Officer)
Date: November 14, 2019
By: /s/ John Docherty
John Docherty
President and Director
Date: November 14, 2019
By: /s/ Allan Spissinger
Allan Spissinger CPA, CA
Chief Financial Officer
(Principal Financial Officer)
Date: November 14, 2019
By: /s/Ted McKechnie
Ted McKechnie
Director
Date: November 14, 2019
By: /s/Nicholas Baxter
Nicholas Baxter
Director
Date: November 14, 2019
By: /s/Brian Quigley
Brian Quigley
Director
Date: November 14, 2019

Page 83 of 83 |