EXHIBIT 99.1
Lexaria Bioscience CEO Discusses Strategic Outlook
Lexaria Bioscience Corp (“Lexaria”, or the “Company”) is a global biotech leader in developing and implementing advanced technologies to better deliver Active Pharmaceutical Ingredients (“APIs”) into the human body for faster and more efficacious use. From humble beginnings with only two patents pending, Lexaria has now been awarded 16 granted patents and has roughly 60 more pending, across different sectors including cannabinoids, nicotine, pharmaceuticals, and vitamins.
Capital Markets
With gratitude to Dickens, “It was the best of times, it was the worst of times…” can aptly be used to review the year recently passed. Lexaria achieved many things during 2019 – indeed, management feels that it achieved virtually all that it had set out to accomplish and it was its best year ever from an operational perspective.
But despite many achievements – which will be reviewed below – a single negative characteristic seemed to overwhelm the psyche if not the reality of the Lexaria community. This of course was the extraordinarily challenging equity markets environment that placed constant pressure upon Lexaria’s valuation, and upon the patience of its many of shareholders.

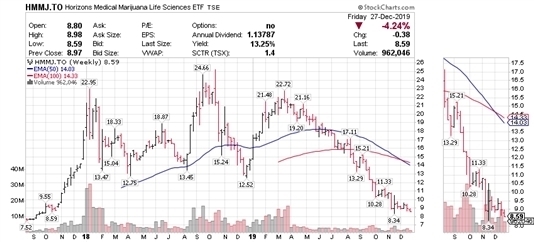
HMMJ ETF
Above is a 2.5 year look at the valuations of the public companies within the cannabis sector, as evidenced by the Horizons Medical Marijuana Life Sciences ETF (“HMMJ”). It is obvious that 2018 was the year of peak valuations; in retrospect it is easy to see that even the highest valuations of 2019 failed to retain the investment public’s enthusiasm of the previous year. Current valuations are much more in line with the depths of 2017 and investors have had to readjust their expectations dramatically. Average valuation declines from the 2019 highs to the recent lows are 63% and many cannabis companies, some of which are not represented in this index, are down 90% - 95%.
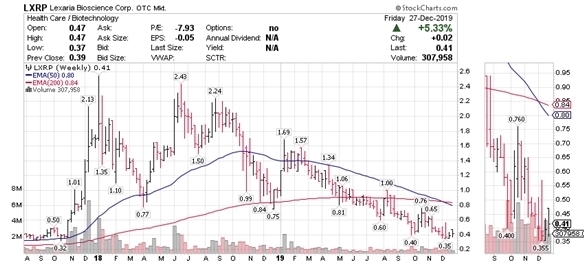
LXRP
Lexaria’s 2.5 year stock chart paints a very similar picture as that of the HMMJ ETF. Volatility commensurate with a small-cap stock is evident, as are similar relative 2018 peaks in valuation and the current trough. Lexaria’s stock price has declined most of the year and is down 79% from the 2019 high.
During 2019 I have personally spoken with many of you, and many more of you have spoken with our investor relations professionals. We’ve heard your concerns as valuations have slipped away and we share those concerns. As the largest shareholder in the Company with well over 13 million shares, I have personally experienced the anguish of a large portfolio loss. I have never sold a share because I knew that doing so would trigger concern in our shareholder base – even though executives and directors in many other public cannabis companies have sold and continue to sell hundreds of millions of dollars-worth of stock in their own companies.
But the main reason I’ve never sold my shares in Lexaria is because I firmly believe the best is yet to come and that Lexaria’s brightest days lie in the future, not in the past. It is my own opinion that Lexaria shares are dramatically undervalued – more so today than at any time in our history. To better understand why I think that, let’s start by reviewing some of the Company’s many recent accomplishments and try to understand how Lexaria’s management team is guiding the Company forward.
Fortune 500 Relationships
Not many small-cap or nano-cap companies ever manage to achieve any kind of official relationship with Fortune 500-style companies. Radically different cultures pervade enormous companies from start-ups; pathways to achieving objectives are rarely the same; and the overwhelming disparity in size makes it difficult for start-up companies with only tens of employees to co-exist alongside large organizations with tens of thousands of employees.
Beginning in the Summer of 2017, Lexaria President John Docherty and I realized that the coming mania in the cannabis sector was going to produce both opportunities and tremendous risk. We commented publicly on the emerging over-capacity in cannabis production fueled by over-aroused dollars seeking fast returns. We pointed out that disruptors like Lexaria could deliver more efficacy with less drug, meaning a 1-million square foot grow facility could be scrapped for a 500,000 square foot facility wherein the cannabis oil output utilized Lexaria’s DehydraTECH.
We started designing Lexaria’s diversification strategy based on core strengths; we felt strongly that our brand-new DehydraTECHTM technology would offer similar benefits to the delivery of nicotine (and potentially many other APIs) as it does to the delivery of cannabinoids. We were also aware of the staggering levels of harm that traditional delivery of nicotine conveyed to its customers and we felt that Lexaria could make a positive impact.
John started designing animal studies along with comments and suggestions received from one of the world’s largest tobacco companies. We published summary information in the Spring of 2018 that evidenced DehydraTECH delivering nicotine more efficiently in oral dosage forms with our tech than without. The tobacco company then helping guide our work then requested that we repeat the study with a larger animal population, to prove that the results from the first study were repeatable. Progress takes time!
As we prepared for the second animal study, summary results of which we press-released in August of 2018, we engaged in serious discussions with Altria Group Inc (“Altria”), owner of both combustible and non-combustible tobacco companies, which have been the undisputed market leaders in the U.S. tobacco industry for decades. The August 2018 press release confirming DehydraTECH’s effectiveness with nicotine delivery roughly coincided with the beginning of our exclusive negotiations with Altria, which were finalized and announced in January 2019.
It took John and I and the rest of the Lexaria task group 18 months to devise our nicotine strategy; design and run initial scientific studies; identify prospective industry partners; and ultimately negotiate with the best of those partners to bring a deal to market. We were overjoyed at our accomplishment, but the enormous potential of this relationship has still not been fully discovered.
We knew from our discussions with some of the world’s most significant tobacco companies that our DehydraTECH technology had the ability to deliver more important results than simply satisfying cannabis users. Some of the largest corporations in the world have asked about our technology. We are confident that, while the cannabis industry was and will remain important to Lexaria, we are now pursuing more significant and more valuable opportunities including positive impacts on public health.
Our strategy of sectoral diversification was correct; we executed it according to plan; and the positive valuation of it is yet to be experienced by our shareholders, offering untold valuation potential both for nicotine and potentially many other APIs beyond cannabinoids.
Lexaria’s workgroup, including extensive ongoing R&D performed by Canada’s National Research Council on our behalf, continues to work closely with Altria to determine appropriateness of the DehydraTECH technology for potential use in the global tobacco industry, via several product formats currently under investigation. Lexaria is making important, significant progress in its goal of achieving reduced-risk methods of nicotine delivery.
It will take additional time to design and gain approval for next-generation nicotine products both within America and around the world. Lexaria is highly confident that it will do so. Lexaria has conducted a number of in-house pre-pilot examinations of its technology utilizing nicotine and I believe we have developed the world’s most advanced oral nicotine delivery system that dramatically reduces the throat-irritation normally associated with oral nicotine delivery while more efficiently delivering nicotine as compared to other technologies. Given the choice, I believe existing adult tobacco consumers will overwhelmingly choose Lexaria-enabled oral nicotine products over competitive products.
As the New Year and new decade begins, Lexaria will be pursuing both long-term and mid-term strategies designed to introduce the benefits of DehydraTECH to North America’s leading companies capable of reaching large segments of each product sector so targeted.
Fresh off our success in attracting Altria, an industry-leading Fortune 500 company that is currently rated 14th on CR Magazine’s list of 100 Best Corporate Citizens and managing that ongoing relationship, Lexaria has been quietly working on additional Fortune 500 opportunities. We have had discussions with leading consumer packaged goods (“CPG”) companies in both Canada and the US throughout 2019, all interested in the potential to utilize DehydraTECH technology within their various CPG product segments.
Whether in the cannabis sector, the various CPG product sectors, the health and wellness sector, or the pharmaceutical sector, Lexaria’s strategy is the same in one key aspect – we formulate and pursue strategies designed to allow for DehydraTECH technology to gain exposure to a minimum 25% market share in each country, in each sector upon which we focus.
Regional or national market share of 25% would be audacious dreaming for a start up, considering the number of different market sectors we are targeting, except for one thing: we’ve already partnered with Altria, a leader in the U.S. tobacco industry. We’ve already demonstrated we can get our technology noticed by companies 1,000 times larger than Lexaria, and we plan to do it again.
DehydraTECH gets noticed because it works. In study after study, positive benefits of rapidity, bioavailability, and positive consumer experience are noted over and over again.
Licensed Laboratory, Intellectual Property, and Facilities
Some of our shareholders were amazed to learn that it was only five months ago – August 2019 – that Lexaria received its first federal government approval to operate a cannabis R&D facility in Canada. How did we accomplish so much in earlier years without such a licensed facility?
In a word: outsourcing. It was often painstakingly slow and sometimes expensive. The head of our scientific team, President John Docherty, would design many programs and studies in vitro (“in the glass”, petri dish or test tube) and in vivo (within a living organism); alter any number of variables; and only then Lexaria would outsource the study to third-party labs and/or industry partners. From an efficiency point of view it wasn’t ideal but it allowed Lexaria to conduct leading research from 2015 to 2019 years before most other companies in the cannabis business had ever started their operations. Lexaria established itself as a world leader in cannabis research.
This, in turn, supported our dozens of new patent applications within the US and around the world. We were one of the first companies in the world to recognize the strategic value of intellectual property (“IP”) filings in the newly emerging cannabis space and we pursued this strategy with vigor. Currently, all of our IP has been developed in-house: we have not yet acquired outside technology, although we may do so in the future.
Meanwhile, our new laboratory has been equipped with state-of-the-art equipment and we are preparing for our busiest R&D year ever, in anticipation of our largest-ever number of new nicotine formulations as part of our ongoing work with Altria.
During Q2 of 2019 we also finalized a new contractual relationship with a GMP-certified, FDA registered production company that has improved our ability to get enhanced DehydraTECH powders to market in the US. Lexaria has placed certain equipment within that facility necessary to perform the DehydraTECH process related to CBD from hemp products. Our current capacity is roughly 200,000 servings per day which can be increased to 400,000 servings per day within the same facility, as needed. One of our goals for 2020 is to utilize the production capabilities of this facility to the greatest extent possible, on behalf of our own internal needs as well as a contracted fee-for-goods and services facility on behalf of our corporate clients.
We also continue our beverage formulation and R&D out of Atlanta, GA; and our pick-and-pack products warehouse, 1-800 call center, and DehydraTECH formulation investigation in Phoenix, AZ.
Lexaria’s IP strategy continues to pay dividends, as we received our first ever patent rights to use DehydraTECH with cannabinoids for treatment of health conditions including Alzheimer’s, Schizophrenia, Parkinson’s, and heart disease, granted in August 2019 in Australia. Lexaria is utilizing the procedural tools offered by the international Patent Prosecution Highway, to try to gain fast-track patent status for these same conditions in the U.S. during 2020. If Lexaria succeeds in gaining patent protection for DehydraTECH not only for composition of matter and formulation method of use patent claims but also specifically for treatment of disease indications in large markets like the US and Europe, we expect the applicability of Lexaria’s technology and valuations could increase dramatically. The Company will for the first time be able to offer pharmaceutical partners specific IP applicability, greatly expanding the value of our IP portfolio.
In September, the results of our human clinical study conducted in 2018 were published in a US peer-reviewed medical journal, “Advances in Therapy”. Advances in Therapy focuses on clinical medicine and pharmaceutical research and has been published continually since 1984. Among other things, this study evidenced that CBD enhanced with DehydraTECH lowered human blood pressure, whereas generic CBD did not. With this publication, Lexaria became one of only a handful of companies to have positive pharmacodynamic results from CBD published in a peer-reviewed journal.
Revenue From Operations
The executive team at Lexaria developed our strategic plan around revenues beginning about three years ago. We know that most biotech companies that are developing and securing their IP portfolio typically experience 5 to 10 years without significant revenues, all the while issuing dilutive equity or often preferred debt instruments to fund operations in the interim. We wanted to avoid the classic biotech equity trap which often results in corporate advancement at the cost of wildly dilutive equity, often leaving original shareholders with a nice story but little per-share gain in value.
Instead, Lexaria developed and continues to implement a multi-pronged strategy to grow its IP portfolio with strategic industry partners, while simultaneously developing quick-to-market revenue strategies that would reduce short term corporate finance needs. From this was borne our concept of licensing our technology within the cannabinoid industries newly emerging in several locations around the world, even while we pursue longer term opportunities in other sectors such as the pharmaceutical industry.
We have not executed this strategy perfectly. At times, companies to which we have licensed our technology have failed to launch their operations or raise their growth capital as expected. Across most of North America, jurisdictions from California to Canada have stumbled as regulators have tried to build a legislative and operational framework that has resulted in repeated delays and tight constraints on business operations. These delays have affected Lexaria as well, since if our corporate clients cannot get their products to market, we cannot generate licensing revenue.
On the other hand, from about mid 2019 onwards I have at times mentioned in media interviews that it was beginning to appear that Q4 2019 and Q1 and Q2 of 2020 were showing signs of a possible turnaround in our revenue generation. As we begin Q1 of 2020, we are seeing signs of increases in revenue to Lexaria that should be sustainable and supportive of future growth.
One of our existing clients is embarking on an aggressive growth strategy from their home state of Colorado, to several additional states within the next year. That brand, 1906 Chocolates, powered by Lexaria’s DehydraTECH, has become one of the most popular in its home state in just three years, originally from a startup with no revenue. Lexaria’s technology helps consumers of 1906 chocolates experience consistent effects, rapidly and predictably, from their cannabis edibles. As Lexaria has stated repeatedly, cannabis companies cannot hope to build brand loyalty with consumers unless their offered brand represents something that consumers value. Generic, slow acting cannabis edibles cannot support brand loyalty. As the 1906 brand expands both geographically and through additional product SKUs, Lexaria expects significant increases in revenue.
Another of our existing clients is just now launching their cold-brewed bottled coffee with CBD. Lexaria has worked diligently with this client for months and is assisting with certain proprietary beverage manufacturing techniques and will be generating initial revenues beginning late in Q1, 2020.
A new client is about to introduce our DehydraTECH technology within their already-successful CBD oral product, across most of the US. Once again, Lexaria has been quietly undergoing formulation development with this client and is about to begin commercial implementation during Q1, 2020.
With these three clients alone, Lexaria expects to more than double its entire 2019 revenue. With additional clients onboarding during 2020, we expect to see additional gains in revenue. We now have enough comfort with working relationships with our corporate clients, to be able to say that 2020 may finally deliver more significant revenues to Lexaria as we work diligently toward profitability.
Positive Trends
There were a number of industry trends and legislative developments in 2019 that are strongly supportive of future growth for Lexaria in 2020. In the US, the SAFE banking act easily passed in the House though it still faces an uncertain future in the Senate. Federally permissible banking for the cannabis industry could do more to encourage growth and sustainability in the industry than any other single development. More recently, Illinois just became the 11th state to legalize cannabis for recreational use – including cannabis edibles - and with the 5th largest state population, created significant new demand for legal cannabis products.
In Canada, more than a full year has passed since cannabis became legal, but only in recent weeks have cannabis edibles been legally available for sale. Legislators in Canada have taken a painstakingly cautious approach to cannabis edibles but the recent availability of legal edibles will spur demand for healthier dosing alternatives to smoking. As well, widely publicized challenges in retail distribution are slowly being solved, with larger numbers of retail cannabis outlets being licensed every week. The recent developments in Canadian edibles legislation represent the first opportunity for consumers in Canada to legally buy and consume cannabis edibles and over time, will drive demand for higher-performing edibles such as those enabled by Lexaria technology.
Arguably the largest cannabis market in North America – California – completely revamped its cannabis laws in January 2019 and for months after, the industry was in disarray as it tried to come to terms with a much stricter regulatory climate. Companies have since learned how to comply and adapt, and 2020 could become the year that new brand leaders emerge and prosper.
In the CBD market, while states like California and Georgia, and the City of New York have all banned CBD as a food additive, the Federal Farm Bill passed in December 2018 for the first time legalized CBD for many uses. Even Major League Baseball dropped its previous prohibitions on natural cannabinoids including THC! Lexaria experienced its strongest interest ever in its technology from companies investigating their own hemp-based products in 2019 and we expect this trend to continue.
Positive developments also occurred in the nicotine industry, where the FDA for the first time in its history allowed certain reduced health-risk claims to be used in association with a type of nicotine product use that serendipitously is closely aligned with product formats amenable to Lexaria’s technology. Reducing or eliminating lung cancers and emphysema through novel forms of nicotine delivery would be a massively positive step in the battle to reduce risk through consumer behavior.
These trends and more all illustrate a common point: Lexaria’s technology is leading edge and at times requires legislative evolution before a commercial market can be developed. DehydraTECH delivers drugs that might otherwise be capable of doing harm and these will always be heavily regulated markets where commercial success includes legislative harmony.
Additional Plans
Lexaria is always busy. Whether in the laboratory or working with our commercial clients, the Company is always working on new formulation ideas and much more. One objective I have for the Company during 2020 is to dramatically alter our capital markets strategy. During the last six months Lexaria has met with a number of Wall Street investment banking firms as we devise our long-term capital markets strategy. We have gained respectful nods from many prominent Wall Street firms, analyst mentions from one of the oldest investment banking firms in the US and analyst coverage from Eight Capital in Canada.
But it all amounts to little more than positive conversation until and unless Lexaria is listed on a recognized US national stock exchange (Nasdaq, Amex, NYSE). Although we are listed on the highest tier available at the OTC (the OTCQX), it is not a nationally recognized stock exchange and many capital and strategic opportunities are closed to us despite our many advancements. Many private equity firms, family offices, and investment dealers are prohibited from investing in companies not listed on a national stock exchange.
Lexaria expects to pursue a strategy during 2020 to qualify for listing on a nationally recognized exchange, allowing a dramatically larger audience of investors to consider Lexaria’s attributes and opening a path to increased quantities and quality of strategic opportunities.
As noted above, Lexaria is also pursuing additional new Fortune 500 strategic partnerships to assist in implementing DehydraTECH technology into consumer brands already established and known. We are currently working towards retail distribution of the Lexaria-owned ChrgD+ branded products into a small network of over 100 retail stores outside of North America. Lexaria has never enjoyed retail distribution in the past and expects revenues would benefit from new retail exposure. Lexaria is currently in trials with potential new clients including a US multi-state operator (“MSO”) in the cannabis industry, wherein DehydraTECH is being evaluated for expanded use. DehydraTECH is also being examined for use by new potential clients in Europe.
Also, a number of public cannabis companies have taken multi-million dollar write-downs on cannabis oil inventories, whether in bulk oil format or in oil filled capsules. These losses are avoidable and represent the lack of knowledge within many newer cannabis companies of available technological options. Lexaria’s DehydraTECH technology converts cannabis oils – with a 12-16 month shelf life – into enhanced processed powders with roughly a 3-year shelf life. Lexaria will continue to try to educate the industry on inventory management techniques that can save cannabis company shareholders millions of dollars.
No strategic view is complete without understanding the people involved. In the over-35 years that I have been in business, I have never experienced the levels of dedication and professionalism that I’ve seen with the people who work for and with Lexaria. There is passion to pursue the goal of making the world a better place. There is purpose in working together towards a common vision. The Lexaria family (you know who you are) is the best I have ever worked with - second to none - and it is my honor to work alongside such fine people.
Summary
2019 was the best year of corporate progress that Lexaria has ever experienced. We started the year with a major announcement with one of the world’s largest companies evaluating our technology, including a negotiated royalty fee applicable to world-wide sales of products utilizing DehydraTECH: our biggest licensing deal ever. We hope to announce ongoing progress with this relationship early in 2020, and we also hope to attract new Fortune 500 CPG licensees in the coming year as well.
Through a partner, we opened a new US facility capable of manufacturing at a potential current rate of up to 200,000 servings of enhanced DehydraTECH powders per day, and we expect this to begin generating licensing as well as fee-for-goods and services revenues for us in early 2020. We signed new corporate clients in the cannabinoid sector throughout the year, many of whom expect to commence retail operations in 2020, and we are increasingly attracting the interest of larger, more established MSOs in the field.
We received approval from Health Canada to open and operate our new cannabis R&D facility in our head office newly-built in Kelowna, Canada, through which we intend to continue to broaden our formulation and IP development and increasingly attract Canadian DehydraTECH licensees with the recent dawn of the legal edibles sector in Canada.
We received our first medical claim patent grants, opening the door to increased likelihood of pharmaceutical operations to come. Lexaria continues to develop its proprietary drug delivery platform and is working on commercial release of DehydraTECH version 2.0 sometime in 2020 with even faster delivery than ever before.
We continue to face headwinds that we need to overcome. We need to evidence wider adoption of our technology within the cannabinoid industries. We also need to provide evidence to the investing public that Lexaria is not a cannabis production company but instead should be distinguished and valued as a leading biotech company with a patented, proprietary drug delivery technology with applications to many industries.
Lexaria expects strong, sustainable revenue growth in 2020 and beyond. We are working towards an application for a national stock exchange listing; hoping to announce additional Fortune-500 relationships; and significant new client relationships.
We are working diligently so that, when we look back on 2020 we remember it only as, “It was the best of times…”
FORWARD-LOOKING STATEMENTS
This release includes forward-looking statements. Statements which are not historical facts are forward-looking statements. The Company makes forward-looking public statements concerning its expected future financial position, results of operations, cash flows, financing plans, business strategy, products and services, competitive positions, growth opportunities, plans and objectives of management for future operations, including statements that include words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions are forward-looking statements, including but not limited to: that any additional patent protection will be realized or that patent achievements will deliver material results; that corporate revenues will increase in 2020 and beyond; that relationships with Fortune 500 businesses may materialize, etc. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that other factors will not affect the accuracy of such forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, capital markets challenges, and other factors which may be identified from time to time in the Company's public announcements and filings. There is no assurance that existing capital is sufficient for the Company's needs or that it will be able to raise additional capital. There is no assurance the Company will be capable of developing, marketing, licensing, or selling edible products containing cannabinoids, nicotine or any other active ingredient. There is no assurance that any planned corporate activity, scientific research or study, business venture, letter of intent, technology licensing pursuit, patent application or allowance, consumer study, or any initiative will be pursued, or if pursued, will be successful. There is no assurance that any of Lexaria’s postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease.
The CSE has not reviewed and does not accept responsibility for the adequacy or accuracy of this release.