
As filed with the Securities and Exchange Commission on January 6, 2021
Registration Number 333-250326
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Amendment No. 1
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
| LEXARIA BIOSCIENCE CORP. |
| (Exact name of registrant as specified in its charter) |
| Nevada |
| 2000 |
| 20-2000871 |
| (State or other jurisdiction of incorporation or organization) |
| (Primary Standard Industrial Classification Code Number) |
| (I.R.S. Employer Identification Number) |
100 – 740 McCurdy Road
Kelowna, BC Canada V1X 2P7
1-250-765-6424
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Christopher Bunka
Lexaria Bioscience Corp.
#100 – 740 McCurdy Road
Kelowna, British Columbia V1X 2P7
1-250-765-6424
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copies to:
| Gregory Sichenzia, Esq. Avital Perlman, Esq. Sichenzia Ross Ference LLP 1185 Avenue of the Americas New York, NY 10036 Telephone: (212) 930-9700 | Robert F. Charron, Esq. Ellenoff Grossman & Schole LLP 1345 Avenue of the Americas New York, NY 10105 (212) 370-1300 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering: ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering: ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering: ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer | ☐ | Accelerated Filer | ☐ |
| Non-Accelerated Filer | ☒ | Smaller Reporting Company | ☒ |
| Emerging Growth Company | ☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered |
| Proposed Maximum Aggregate Offering Price(1) |
|
| Amount of Registration Fee |
| ||
| Common stock, par value $0.001 per share(2)(3) |
| $ | 9,200,000 |
|
| $ | 1,003.72 |
|
| Warrants to purchase common stock(4) |
|
| — |
|
|
| — |
|
| Common stock issuable upon exercise of warrants(2)(3) |
| $ | 13,800,000 |
|
| $ | 1,505.58 |
|
| Pre-funded warrants to purchase common stock |
|
| (5 | ) |
|
| — |
|
| Common stock issuable upon exercise of pre-funded warrants |
| $ | (5 | ) |
| $ | - |
|
| Representative’s warrants to purchase common stock(4) |
|
| — |
|
|
| — |
|
| Shares of common stock issuable upon exercise of representative’s warrants(2)(3)(6) |
| $ | 920,000 |
|
| $ | 100.38 |
|
| Total |
| $ | 23,920,000 |
|
| $ | 2,609.67 | (7) |
_________________
| (1) | Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended (the “Securities Act”). |
| (2) | Pursuant to Rule 416, the securities being registered hereunder include such indeterminate number of additional securities as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. |
| (3) | Includes additional shares of common stock and/or warrants to purchase shares of common stock (and the shares of common stock issuable thereunder) representing 15% of the number of shares of common stock (including the number of shares of common stock issuable upon exercise of the pre-funded warrants)and the number of warrants to purchase common stock which may be offered to the public, that the underwriter has the option to purchase. |
| (4) | No fee required in accordance with Rule 457(g) under the Securities Act. |
| (5) | The proposed maximum aggregate offering price of the common stock will be reduced on a dollar-for-dollar basis based on the offering price of any pre-funded warrants sold in the offering, and the proposed maximum aggregate offering price of the pre-funded warrants to be sold in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any common stock sold in the offering. Accordingly, the proposed maximum aggregate offering price of the common stock and pre-funded warrants (including the common stock issuable upon exercise of the pre-funded warrants), if any, is $9,200,000, including the underwriter’s option to purchase additional shares of common stock. |
| (6) | Represents warrants to purchase a number of shares of common stock equal to 8% of the number of shares of common stock sold in this offering, including shares of common stock issuable upon exercise of the pre-funded warrants, and including upon exercise of the option to purchase additional shares of common stock, at an exercise price equal to 125% of the public offering price per share. |
| (7) | $2,107.82 previously paid. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said section 8(a), may determine.
| i |
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JANUARY 6, 2021
PRELIMINARY PROSPECTUS

1,066,666 Shares of Common Stock
Pre-Funded Warrants to purchase up to 1,066,666 Shares of Common Stock
Warrants to purchase up to 1,066,666 Shares of Common Stock
We are offering for sale 1,066,666 shares of our common stock, par value $0.001 per share. Each share of our common stock is being sold with one warrant to purchase one share of our common stock, at an assumed combined public offering price of $7.50 per share of common stock and related warrant, the closing sale price per share of our common stock on the OTCQX on December 31, 2020, on a post reverse stock split basis, representing a public offering price of $7.499 per share of common stock and $0.001 per related warrant. The warrants are exercisable on the date of issuance and expire on the five year anniversary of such date, at an exercise price per share of common stock equal to $______. The shares of common stock and warrants can only be purchased together in this offering but the shares of common stock and warrants are immediately separable and will be issued separately.
We are also offering to those purchasers, if any, whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, pre-funded warrants in lieu of shares of common stock that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant is being sold with one warrant to purchase one share of our common stock, at an assumed combined public offering price of $7.499 per pre-funded warrant and related warrant, the closing sale price per share of our common stock on the OTCQX on December 31, 2020, less the $0.001 exercise price of the pre-funded warrant). The exercise price of each pre-funded warrant will equal $0.001per share. Each pre-funded warrant will be exercisable on the date of issuance and will not expire prior to exercise. The pre-funded warrants and warrants can only be purchased together in this offering, but the pre-funded warrants and warrants are immediately separable and will be issued separately. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. Because we will issue a warrant for each share of our common stock and for each pre-funded warrant sold in this offering, the number of warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold.
Our common stock is quoted on the OTCQX under the symbol “LXRP” in the United States and trades on the Canadian Securities Exchange (CSE) under the symbol “LXX” in Canada. On January 5, 2021, the closing price per share of our common stock as quoted on the OTCQX was $0.25 per share and as traded on the CSE was CDN$0.33 per share ($7.50 and CDN$9.90, respectively, on a post reverse stock split basis).
| ii |
We are in the process of applying to have our common stock and warrants sold in this offering listed on the Nasdaq Capital Market (“Nasdaq”) under the symbols “LEXX” and “LEXXW”, respectively. There is no assurance that our listing application will be approved. In connection with our listing application, we will effect the reverse stock split of our common stock at a ratio of 1-for-30. We will not consummate this offering unless our shares of common stock and warrants are approved for listing on Nasdaq. We have not applied to list the warrants on the CSE. After this offering, our common stock will continue to trade on the CSE.
Unless otherwise noted and other than in our financial statements in this registration statement and the notes thereto, the share and per share information in this prospectus reflects the anticipated reverse stock split of the outstanding common stock of the Company at an assumed 1-for-30 ratio, which split will occur concurrently with the pricing of the offering.
The combined public offering price per share (or pre-funded warrant) and related warrant will be determined between us, the underwriters and investors based on market conditions at the time of pricing, and may be at a discount to the current market price of our common stock. Prior to this offering, there has been a limited public market for our common stock on the OTCQX. Therefore, the assumed combined public offering price used throughout this prospectus may not be indicative of the actual combined public offering price.
Investing in our securities involves a high degree of risk. Before buying any securities, you should read the discussion of material risks of investing in our common stock under the heading “Risk Factors” beginning on page 8 of this prospectus.
Neither the Securities and Exchange Commission nor any foreign or state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
|
|
| Per Share and Warrant combination |
| Per |
|
| Total |
| ||
| Public offering price(1) |
| $ |
| $ |
|
| $ |
| ||
| Underwriting discounts and commissions(2) |
| $ |
| $ |
|
| $ |
| ||
| Proceeds to us (before expenses) |
| $ |
| $ |
|
| $ |
| ||
(1) The assumed public offering price and underwriting discounts and commissions correspond to (x) in respect of a share of common stock and related warrant, (i) a public offering price per share of common stock of $7.499 and (ii) a public offering price per warrant of $0.001 and (y) in respect of a pre-funded warrant and related warrant (i) a public offering price per pre-funded warrant of $7.498 and (ii) a public offering price per warrant of $0.001.
(2) We have agreed to pay the representative of the underwriters a management fee equal to 1.0% of the gross proceeds of this offering, to issue to the representative warrants to purchase shares of common stock equal to 8% of the shares issued in this offering (including shares underlying the pre-funded warrants) and to reimburse certain expenses of the representative in connection with this offering. See “Underwriting” beginning on page 83 for additional disclosure regarding the compensation payable to the underwriters.
| iii |
We have granted the underwriters an option to purchase an additional 159,999 shares of common stock and/or warrants to purchase 159,999 additional shares of common stock from us (being up to 15% of the shares of common stock, including shares of common stock underlying the pre-funded warrants, and/or up to 15% of the warrants sold in this offering), in any combination thereof, at the public offering price per share and public offering price per warrant, respectively, less the underwriting discounts and commissions, for thirty (30) days from the date of this prospectus.
No shares of common stock, pre-funded warrants or warrants will be offered or sold in Canada, including through the CSE or any other trading market in Canada, in this offering.
Delivery of the shares of common stock, the pre-funded warrants, if any, and the warrants is expected to be made on or about ________, 2021, subject to satisfaction of customary closing conditions.
H.C. WAINWRIGHT & CO.
The date of this prospectus is ________, 2021.
| iv |
|
|
| 1 |
| |
|
|
| |||
|
|
| 8 |
| |
|
|
| |||
|
|
| 32 |
| |
|
|
| |||
|
|
| 33 |
| |
|
|
| |||
|
|
| 33 |
| |
|
|
| |||
|
|
| 34 |
| |
|
|
|
|
|
|
|
|
| 36 |
| |
|
|
|
|
|
|
|
|
| 38 |
| |
|
|
| |||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
|
| 40 |
|
|
|
| |||
|
|
| 50 |
| |
|
|
| |||
|
|
| 73 |
| |
|
|
| |||
|
|
| 77 |
| |
|
|
| |||
|
|
| 82 |
| |
|
|
| |||
|
|
| 82 |
| |
|
|
| |||
|
|
| 83 |
| |
|
|
| |||
|
|
| 88 |
| |
|
|
| |||
|
|
| 94 |
| |
|
|
| |||
|
|
| 94 |
| |
|
|
| |||
|
|
| 95 |
| |
|
|
| |||
|
|
| F-1 |
|
| v |
About This Prospectus
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. The information contained in this prospectus is accurate only as of the date of this prospectus. Our business, financial condition, results of operations and prospects may have changed since such date. Other than as required under the federal securities laws, we undertake no obligation to publicly update or revise such information, whether as a result of new information, future events or any other reason.
The distribution of this prospectus and the offering of our securities in certain jurisdictions may be restricted by law. This prospectus does not constitute, and may not be used in connection with, an offer or solicitation by anyone in any jurisdiction in which such offer or solicitation is not authorized or in which the person making such offer or solicitation is not qualified to do so to any person to whom it is unlawful to make such offer or solicitation. See the “Underwriting” section of this prospectus beginning on page 83. In this prospectus, references to “the Company,” “we,” “us,” and “our” refer to Lexaria Bioscience Corp. Unless otherwise specified, all dollar amounts are expressed in United States dollars. All references to "CDN$" refer to Canadian dollars.
Purchasers of securities hereunder are advised that none of the securities offered hereunder will be qualified for distribution in any jurisdiction of Canada, and may not be traded through the facilities of the CSE or any other Canadian stock exchange, or otherwise in a jurisdiction of Canada. By purchasing securities hereunder, each purchaser thereof will be deemed to have represented and warranted to the Company and the underwriters that such purchaser (i) is acquiring the securities solely for its own account and beneficial interest for investment purposes, and not for sale or with a view to distribution in Canada, and (ii) has no present intention of selling the securities through the facilities of the CSE or any other Canadian stock exchange, or otherwise in a jurisdiction of Canada, and does not presently have any reason to expect a change in such intention.
| vi |
| Table of Contents |
This summary provides an overview of selected information contained elsewhere in this prospectus and does not contain all of the information you should consider before investing in our securities. You should carefully read this prospectus and the registration statement of which this prospectus is a part in their entirety before investing in our securities, including the information discussed under “Risk Factors” beginning on page 8 and our historical and pro forma financial statements and notes thereto that appear elsewhere in this prospectus.
Business Overview
General and Historical Overview of Our Business
The Company was formed on December 9, 2004 under the laws of the State of Nevada. In March of 2014, the Company began work in the fields of enhanced delivery of active ingredients and drugs. The Company filed its first patent application in 2014 and today has over 50 patent applications pending around the world, with 18 patents granted to date, all related to its DehydraTECHTM technology (“DehydraTECH” or the “Technology”) and certain characteristics of oral ingredient and drug delivery. Additional early stage investigation has been conducted of topically-administered products such as patches, creams and lotions using DehydraTECH.
The Company’s patent applications developed from its Research and Development programs (“R&D”) which currently include fat-soluble versions of vitamins, NSAIDs, nicotine, cannabinoids, hormones, phosphodiesterase inhibitors, and antivirals. Our 2018 animal studies demonstrated a propensity for the DehydraTECH technology to elevate the quantity of nicotine delivered across the blood-brain-barrier. The results discovered from our animal studies led to an expansion of our patent applications and opened possibilities for improved delivery of certain central nervous system-targeted drugs that require additional R&D.
In a 12-participant human clinical study performed in 2018 and published in 2019 in a peer reviewed medical journal, Advances in Therapy titled “Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study” available on the PubMed.gov website with the identification of PMID: 31512143, the Company demonstrated that its technology delivered higher volumes of cannabidiol into the human circulatory system and did so more quickly than a concentration-matched positive control. This same study also demonstrated a statistically significant reduction in human blood pressure from the DehydraTECH processed cannabidiol, versus no statistical reduction in human blood pressure from the positive control.
In the Company’s most recent animal study conducting research on DehydraTECH within two classes of antiviral therapies (a Protease Inhibitor and a Revers e Transcriptase Inhibitor) which are currently in use against HIV/AIDS and are being investigated for use against SARS-CoV-2/COVID-19, noted improvements in the delivery of the antiviral drugs in the animal bloodstream were demonstrated. The improved delivery of the antivirals along with the animal’s demonstrated safety and tolerability of the DehydraTECH formulations has led the Company to begin preparations for expanded investigations into antiviral drug delivery enhancement and effectiveness and filing additional patent applications.
We operate a Health Canada-licensed laboratory located in Kelowna, British Columbia, Canada to conduct basic research and formulation operations, and typically outsource all analytical work to independent third-party laboratories located in Canada, the USA, and Europe. Such third-party evaluation provides independent assessment of the effects of our technology and processes. We aim to partner with industry leaders for adoption of DehydraTECH into their consumer products and/or drugs. Other than for R&D purposes, the Company does not produce, manufacture, market or distribute drugs.
| 1 |
| Table of Contents |
Our Current Business
Our current business plan is focused on the development of strategic partnerships with licensees for our patented DehydraTECH™ technology in exchange for up front and/or staged licensing fees over time. In addition, we continue to investigate national and international opportunities for development and distribution of the Company’s DehydraTECH enhanced oral and topical product offerings; to investigate expansions and additions to our intellectual property portfolio; and to search for additional opportunities in alternative health sectors. This includes the acquisition or development of intellectual property if and when we believe it is advisable to do so.
Our current patent portfolio includes patent family grants relating to: (i) Food and Beverage Compositions Infused with Lipophilic Active Agents and Methods of Use Thereof; (ii) Methods for Formulating Orally Ingestible Compositions Comprising Lipophilic Active Agents; and (iii) Stable Ready-To-Drink Beverage Compositions Comprising Lipophilic Active Agents, all pertaining to the Company’s method of improving bioavailability and taste, and the use of the Technology as a delivery platform for a wide variety of Active Pharmaceutical Ingredients (“APIs”) including, but not limited to, fat soluble vitamins; NSAIDs pain medications; and nicotine.
We believe that DehydraTECH can deliver nicotine through orally delivered products that would dramatically reduce the incidence of pulmonary diseases caused by smoking. The Company is aggressively pursuing patent protection in various countries around the world. The Company currently has more than 50 patent applications pending worldwide and, due to the complexity of pursuing patent protection, the quantity and status of patent applications will vary continuously as each application advances or stalls in the examination process. We have filed and intend to continue to file new patent applications for new discoveries that arise from our R&D programs however, due to the inherent unpredictability of scientific discovery, it is not possible to predict if or how often such new applications might be filed.
Our Current Business Developments
The Company is currently focused on the development and improvement of DehydraTECH, as well as the licensing of DehydraTECH to companies operating in the nicotine, pharmaceutical, nutraceutical and vitamin industries. The following events represent our current business developments.
On March 19, 2020, the Company announced that it intended to commence a program to conduct tests to research the benefits of DehydraTECH in connection with enhancing the delivery of certain antiviral drugs.
On April 21, 2020, the Company announced the filing of a new US patent application under a new patent family “Compositions and Methods for Enhanced Delivery of Antiviral Agents” to utilize DehydraTECH process in connection with antiviral drugs for the purposes of combatting infectious disease conditions potentially including, but not limited to, the novel coronavirus disease 2019 (“COVID-19”), MERS, SARS, influenza, herpes and AIDS.
On May 4, 2020, the Company entered into agreements with certain investors for the sale of 295,540 shares of common stock and warrants to purchase up to 295,540 shares of common stock for gross proceeds of $2,039,229. The warrants have a five-year term and are exercisable at $10.20 per share. The financing closed in two tranches on May 6, 2020 and May 11, 2020 with $600,000 of the proceeds being allocated towards funding DehydraTECH R&D and patent application filings.
On June 29, 2020, the Company issued 11,574 shares of restricted common stock to IRTH Communications, LLC (“IRTH”) bearing a deemed aggregate value of $100,000 or $8.64 per share as partial compensation for investor relations services to be provided to the Company. In addition, the Company has also agreed to pay IRTH a cash fee of $7,500 per month for the Services and may, at its sole discretion, engage IRTH to provide additional services at additional costs.
| 2 |
| Table of Contents |
On July 21, 2020, the Company announced filing an application with a national securities exchange in the United States to request an uplisting of the Company’s common stock.
On July 28, 2020, the Company announced that it has received ethics board approval by a European university research hospital to conduct an exploratory clinical study using CBD formulated together with its patented DehydraTECH™ technology to assess blood pressure reduction potential in volunteers with pre- or mildly- hypertensive, middle-aged volunteers.
On August 31, 2020, the Company announced a research and development (“R&D”) framework agreement with British American Tobacco (Investments) Limited (“BAT”) to investigate the Company’s technology for potential use in nicotine products. R&D work under the Agreement will be paid for by BAT.
On September 22, 2020, the Company announced that U.S. Patent No. 10,756,180 was granted; it has claims that protect the use of its DehydraTECH technology together with cannabinoids, nicotine, nonsteroidal anti-inflammatory drugs, or vitamins in mix and serve beverage formats. The patent is entitled “Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof”.
On December 2, 2020, the Company announced that its DehydraTECH technology significantly improved delivery in study animals of representative drugs from two classes of antiviral therapies (a Protease Inhibitor and a Reverse Transcriptase Inhibitor) under investigation against SARS-CoV-2/COVID-19 and already in use against HIV/AIDS. The study animals were not infected with or treated for any diseases. These are the first two of a series of antiviral drugs to be tested using Lexaria’s DehydraTECH technology.
| Drug | Drug Class | AUClast* Delivery & Improvement (hr∙ng/mL) | Control (hr∙ng/mL) | AUC∞** Delivery & Improvement (hr∙ng/mL) | Control (hr∙ng/mL) |
|
Darunavir |
Protease Inhibitor |
721 ± 332 54% (p=0.036) |
469 ± 252 |
726 ± 211 35% (p=0.062) |
536 ± 223 |
|
Efavirenz |
Non-nucleoside Reverse Transcriptase Inhibitor |
752 ± 203 16% (p=0.11) |
650 ± 148 |
1072 ±40 42% (p=0.028) |
757 ±103 |
Our Historical Business Developments
Historically, the Company had focused its DehydraTECH R&D on the combination of DehydraTECH with cannabinoids. While the Company has never been involved in the production or cultivation of cannabis, DehydraTECH has been historically licensed to companies that operate in the cannabis industry. Pursuant to this historical business framework, the following events represent the material business developments.
On or around October 21, 2019, the Company submitted an amendment to its Health Canada research license, which was originally granted on August 9, 2019, to allow for human organoleptic sensory testing. The amendment to the license was approved by Health Canada on June 8, 2020 and remains effective until August 9, 2023.
On January 14, 2020, the Company announced that it had entered into a definitive 10-year agreement, via its subsidiary Lexaria Hemp Corp., with Boldt Runners Corporation (dba Cannadips) to provide DehydraTECH on an exclusive basis in the U.S. for use in oral pouches containing CBD and less than 0.29% THC.
On January 22, 2020, the Company announced that it had entered into a definitive 10-year agreement, via its wholly-owned subsidiary Lexaria CanPharm ULC (“CanPharm”), with Trinidad Consulting LLC (dba Cannadips) to license DehydraTECH on an exclusive basis in the U.S. for use in oral pouches containing over 0.3% THC. This license was subsequently assigned to Hill Street Beverage Company Inc. (“Hill Street”) on November 18, 2020 pursuant to the sale of CanPharm’s license to use and sublicense DehydraTECH for use in the production of consumer products containing over 0.3% THC - see Disposition of Certain Assets below.
On February 26, 2020, the Company terminated the agreement, entered into by its subsidiary Lexaria Hemp Corp., to provide DehydraTECH to a Nevada-based private company for its utilization of DehydraTECH in certain CBD-based beverages, due to lack of performance by the licensee.
On November 18, 2020, the Company announced that it had entered into a definitive 10-year agreement, via its subsidiary Lexaria Hemp Corp., with Hill Street to provide DehydraTECH on a non-exclusive global basis for use in multiple CBD-infused products. This agreement replaces a previous license agreement and joint venture agreement between Lexaria Hemp Corp. and Hill Street as originally announced on July 23, 2019.
| 3 |
| Table of Contents |
On March 4, 2020, the Company announced that it had amended its license agreement with Universal Hemp LLC, a B2B manufacturing company of hemp-derived bulk ingredients, to remove the exclusivity rights originally associated with the license and to reduce the aggregate minimum performance fees from $3,750,000 to $132,500.
Disposition of Certain Assets
The Company is redirecting its business focus away from licensing DehydraTECH to cannabis companies and towards companies that operate in the nicotine, pharmaceutical, nutraceutical and vitamin industries. In order to facilitate this change in business strategy, on November 18, 2020, CanPharm entered into an asset purchase agreement with Hill Street, for the sale of CanPharm’s rights to use and sub-license DehydraTECH with respect to the research, manufacture, assembly, marketing, sale and distribution of cannabis-related products containing 0.3% or greater THC, which includes the assignment of CanPharm’s right as the licensee of DehydraTECH and the assignment of CanPharm’s right as the licensor of third party license agreements with US and Canadian third party cannabis companies that are using or plan to use DehydraTECH within their cannabis-related products (the “CanPharm Asset Sale”). Also included in the sale are SOPs (Standard Operating Procedures), recipes, processes, formulations and techniques. The CanPharm Asset Sale and the transactions contemplated therein closed on December 9, 2020 . As of the closing on December 9, 2020 , the Company and CanPharm no longer have a part or interest in those ongoing revenue streams from the license agreements. As consideration for the assets sold by CanPharm to Hill Street in the CanPharm Asset Sale, Hill Street has (i) paid CanPharm CDN$350,000 on closing, (ii) agreed to issue to CanPharm CDN$1,500,000 of common shares of Hill Street (“Hill Street Shares”), of which 6,031,363 Hill Street Shares valued at CDN$500,000 were issued at closing and the remaining balance shall be issued in two separate tranches of CDN$500,000 on the eight (8) month anniversary and sixteen (16) month anniversary of closing of the CanPharm Asset Sale, respectively, and (iii) issued to CanPharm a promissory note having a principal amount of CDN$2,000,000 and bearing interest at a rate of 10% per annum. The promissory note has no maturity date and is to be paid from licensing and product revenues received by Hill Street from its use or sublicense of DehydraTECH.
Listing on the Nasdaq Capital Market
Our common stock is currently quoted on the OTCQX and trades on the CSE. In connection with this offering, we are in the process of applying to have our shares of common stock and warrants sold in this offering listed on Nasdaq under the symbols “LEXX” and “LEXXW”, respectively. If our listing application is approved, we expect to list our common stock and warrants on the Nasdaq upon consummation of the offering, at which time our common stock will cease to be quoted on the OTCQX. Nasdaq’s listing requirements include, among other things, a minimum stock price requirement. As a result, on or prior to effective date of the registration statement for this offering, we will be required to take the necessary steps to meet the Nasdaq’s listing requirements, including but not limited to a reverse split of our outstanding common stock. If Nasdaq does not approve the listing of our common stock and warrants, we will not consummate this offering. There can be no assurance that our common stock and warrants will be listed on Nasdaq. We have not applied to list the warrants on the CSE. After this offering, our common stock will continue to trade on th e CSE.
| 4 |
| Table of Contents |
Reverse Stock Split
On June 23, 2020, our stockholders approved a reverse stock split within the range of 1-for-2 to 1-for-30 of our issued and outstanding shares of common stock and authorized the board of directors (the “Board”), in its discretion, to determine the final ratio, effective date, and date of filing of the certificate of amendment to our articles of incorporation in connection with the reverse stock split. The purpose of the reverse stock split is to meet Nasdaq’s minimum stock price requirement. The reverse stock split will not change the number of authorized shares of common stock which will remain at 220,000,000 shares. Unless otherwise noted, the share and per share information in this prospectus reflects, other than in our financial statements and the notes thereto, an anticipated reverse stock split of the outstanding common stock of the Company at an assumed 1-for-30 ratio which will occur concurrently with the pricing of the offering.
Impact of COVID-19
The presence of COVID-19 in over 220 countries around the world presents significant and unforecastable new risks to the Company and its business plan. Restrictions on national and international travel, and required business closures, have made it increasingly difficult to carry out normal business activities related to corporate finance efforts and customer acquisition. As a result, the COVID-19 pandemic will almost certainly increase risks of lower revenues and higher losses for the products and services currently offered by the Company. We are monitoring our licensees and are working with them, where possible, to prevent contract default and contract terminations. In some cases, we, in accordance with the relevant governing agreements, have issued termination of contract notices to some of our licensees. These terminations have resulted in $50,000 in write offs of accounts receivable for the year ended August 31, 2020.
The Company is encountering significant challenges in executing its business plan and normal business operations as a result of COVID-19 and does not currently have sufficient resources to withstand a protracted term during which most business activities are curtailed. We have implemented cost containment initiatives to reduce operating expenses and preserve cash that include dismissal of one employee, termination of contracts with two consultants and reduction of compensation payable to certain other consultants as a result of the COVID-19 pandemic. We have not had to close operations or locations as our contractors and staff can work remotely and our third-party fulfillment centers continue to operate.
We have not experienced any significant impacts on our material supply chains but have noted increased timelines from some third-party research facilities regarding their ability to conduct research and testing. To date, this has not significantly impacted our R&D programs, but we cannot predict whether our R&D programs will be impacted in the future.
On March 19, 2020, as noted under Our Current Business Developments, the Company announced that it intended to commence a program to conduct tests to research the benefits of DehydraTECH in connection with enhancing the delivery of certain antiviral drugs. The tests are intended to include a pilot human pharmacokinetic exploratory study in healthy volunteers of two antiviral drugs that had previously been studied against other coronavirus strains, comparing DehydraTECH formulations to controls without DehydraTECH. The Company intends to conduct the study at a leading Canadian university where a study design and plan was submitted and ethics board approval was received. The study is subject to further government regulatory approval. The Company is currently in the process of pursuing the necessary steps to file for study approval from Canadian federal regulators.
| 5 |
| Table of Contents |
In parallel, the Company launched a separate rodent antiviral study to evaluate pharmacokinetic benefits from the use of DehydraTECH in the delivery of representative drugs from two classes of antiviral drugs under investigation for treatment of COVID-19. The results of that animal study were released on December 1, 2020 whereby the DehydraTECH enhanced antiviral drug formulations demonstrated increased delivery of the antiviral drugs into the bloodstream of the animals. The results of this animal study have encouraged the Company to conduct expanded investigations into antiviral drug delivery enhancement, with such investigations including remdesivir (a nucleotide reverse transcriptase inhibitor); as well as three additional drugs known to target the main protease associated with SARS-CoV-2 infection . The Company intends to make its research results available to researchers throughout the world looking to maximize the effectiveness of their own drug investigations. The Company’s business model relies on performing early stage studies like these to help support its efforts to form commercial relationships with more established companies.
The Company continues to monitor governmental programs being released to assist with the COVID-19 pandemic.
Corporate Information
Our common stock is quoted on the OTCQX under the symbol “LXRP” and trades on the CSE under the symbol “LXX”.
Our principal executive offices are located at #100 – 740 McCurdy Road, Kelowna, British Columbia V1X 2P7, and our telephone number is +1 (250) 765-6424. We have administrative functions located in Phoenix, Arizona. Our main corporate website is located at www.lexariabioscience.com. The information on our website is not incorporated by reference into this prospectus.
Due to the implementation of British Columbia Instrument 51-509 on September 30, 2008, by the British Columbia Securities Commission (“BCSC”), we are considered a British Columbia based reporting issuer. As such, we are required to file certain information and documents at www.sedar.com.
The Offering
| Common stock offered by us |
| 1,066,666 shares. |
|
|
|
|
| Warrants offered by us |
| Warrants to purchase up to 1,066.666 shares of common stock, which will be exercisable during the period commencing on the date of their issuance and ending five years from such date at an exercise price per share of common stock equal to $_____. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the warrants. |
|
|
|
|
| Pre-funded warrants offered by us |
| We are also offering to those purchasers, if any, whose purchase of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, pre-funded warrants in lieu of shares of common stock that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant is being sold with one warrant to purchase one share of our common stock, at an assumed combined public offering price of $7.499 per pre-funded warrant and related warrant, the closing sale price per share of our common stock on the OTCQX on December 31, 2020, representing a public offering price of $7.498 per pre-funded warrant and $0.001 per related warrant. The pre-funded warrants and warrants are sold as a fixed combination hereunder but will be issued separately. The exercise price of each pre-funded warrant will equal $0.001 per share. Each pre-funded warrant will be exercisable upon issuance and will not expire prior to exercise. We are offering up to 1,066,666 shares of common stock and up to 1,066,666 pre-funded warrants, in the aggregate not exceeding more than a total of 1,066,666 shares of common stock and pre-funded warrants. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. This prospectus also relates to the offering of the shares of our common stock issuable upon exercise of the pre-funded warrants. |
|
|
|
|
| Underwriter’s Option to purchase additional shares |
| We have granted the underwriters an option to purchase up to an additional 159,999 shares of common stock and/or warrants to purchase up to 159,999 shares of common stock, in any combination thereof, from us at the public offering price per share and per warrant, less the underwriting discounts and commissions (up to 15% of the number of shares of common stock, including shares underlying the pre-funded warrants, and/or 15% of the number of warrants in this offering), for a period of 30 days from the date of this prospectus. |
|
|
|
|
| Common stock outstanding after this offering |
| 4,068,143 shares(1) (assuming we sell only shares of common stock, assuming no exercise of the underwriters’ option to purchase additional shares of common stock and/or additional warrants, and assuming no exercise of the warrants). |
| 6 |
| Table of Contents |
| Use of proceeds |
| The net proceeds from our sale of shares of our common stock in this offering will be approximately $6,863,093, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their option in full to purchase additional shares of common stock and additional warrants, our net proceeds from this offering will be approximately $7,955,093. We plan to use approximately $2,560,000 of the net proceeds of this offering ($3,700,000 if the underwriters exercise their option to purchase additional securities in full) for research and development studies and the patent and legal costs associated thereto , with the remaining net proceeds to be used for general working capital purposes. For additional information please refer to the section entitled “Use of Proceeds” on page 33 of this prospectus. |
|
|
|
|
| Risk Factors |
| Investing in our securities involves a high degree of risk. You should carefully review and consider the “Risk Factors” section of this prospectus for a discussion of factors to consider before deciding to invest in shares of our common stock. |
|
|
|
|
| Representative’s Warrants |
| We will issue to the representative of the underwriters, or its designees, at the closing of this offering common stock purchase warrants to purchase the number of shares of common stock equal to 8% of the aggregate number of shares of common stock sold in this offering, including shares underlying the pre-funded warrants sold in this offering and including shares sold upon exercise of the option to purchase additional securities. The representative’s warrants will be exercisable immediately and will expire five years after the effective date of the registration statement for this offering and are registered on the registration statement of which this prospectus is a part. The exercise price of the underwriters’ warrants will equal 125% of the public offering price per share. See “Underwriting.” |
|
|
|
|
| Proposed Nasdaq listing and symbol
|
| Our common stock is quoted on the OTCQX under the symbol “LXRP” and trades on the CSE under the symbol “LXX.” On January 4, 2021, the last reported sale price of our common stock on the OTCQX and CSE was $0.268 per share and $CDN 0.345 per share, respectively. We are in the process of applying to have our shares of common stock and warrants listed on Nasdaq under the symbols “LEXX” and “LEXXW”, respectively. We do not intend to apply to list the pre-funded warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants will be limited. |
|
|
|
|
| Reverse Stock Split |
| On June 23, 2020, our stockholders approved a reverse stock split within the range of 1-for-2 to 1-for-30 of our issued and outstanding shares of common stock and authorized the Board, in its discretion, to determine the final ratio, effective date, and date of filing of the certificate of amendment to our articles of incorporation in connection with the reverse stock split. In connection with our listing application, we will effect the reverse split of our common stock at a ratio of 1-for-30 concurrently with the pricing of the offering. Unless otherwise stated and other than in our financial statements and the notes thereto, all share and per share information in this prospectus reflects an anticipated reverse stock split of the outstanding common stock and treasury stock of the Company at an assumed 1-for-30 ratio which will occur concurrently with the pricing of the offering. |
(1) The number of shares of common stock that will be outstanding after this offering set forth above is based upon 3,001,477 shares of common stock outstanding as of January 4, 2021, and gives effect to our planned reverse stock split at an assumed ratio of 1-for-30, but does not include, as of that date:
|
| · | 439,095 shares of common stock issuable upon the exercise of outstanding warrants; |
|
|
|
|
|
| · | 170,267 shares of common stock issuable upon the exercise of outstanding stock options; |
|
|
|
|
|
| · | no exercise of the warrants; |
|
|
|
|
|
| · | no exercise by the underwriters of their option to purchase additional shares of common stock and/or warrants to purchase common stock; and |
|
|
|
|
|
| · | no exercise by the underwriters of the underwriters’ warrants issued in this offering. |
|
|
|
|
|
| • | the audited 10-k included in section F has not been updated for the planned reverse stock split |
| 7 |
| Table of Contents |
You should carefully consider the risks described below as well as other information provided to you in this document, including information in the section of this document entitled “Forward Looking Statements”. If any of the following risks actually occur, our business, financial condition or results of operations could be materially adversely affected, the value of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Current Business
Much of the information included herein includes or is based upon estimates, projections or other "forward looking statements". Such forward-looking statements include any projections or estimates made by us and our management in connection with our business operations. While these forward-looking statements, and any assumptions upon which they are based, are made in good faith and reflect our current judgment regarding the direction of our business, actual results will almost always vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested herein.
In developing DehydraTECH, we rely upon our employees, contractors, consultants and collaborators and other third-party relationships, including the ability to obtain appropriate product liability insurance. There can be no assurance that this reliance and these relationships will continue as required. In addition to developmental and operational considerations, market prices for securities of biotechnology companies generally are volatile and may or may not move in a manner consistent with the progress we have made or are making. Prospects for companies in the biotechnology industry generally may be regarded as uncertain given the nature of the industry and, accordingly, investments in biotechnology companies should be regarded as speculative.
Because there is no assurance that we will generate material revenues, we face a high risk of business failure.
There can be no assurance that we will achieve significant revenues or profitable operations or will generate adequate funds to continue our intellectual property development. Many factors (e.g. competition, patent protection, appropriate regulatory approvals) can influence the revenue and our profitability potential. We cannot be sure that our overall business model within any particular sector will ever come to fruition, and if they do, will not decline over time. We may not recover all or any portion of our capital investment in our research and technology development, marketing, or other aspects of the business. Although we exercise due consideration in our development of our technology, ultimately consumer acceptance of our licensees’ products is not reliably forecastable.
In addition, our intellectual property and technology development plans may be curtailed, delayed or cancelled as a result of lack of adequate capital and other factors, such as weather, pandemics, compliance with governmental regulations, current and forecasted prices for input costs of research materials and changes in the estimates of costs to complete the projects. You should understand that our research plans are subject to change.
Our revenues are primarily generated from our out-licensing of DehydraTECH. We should be considered a start-up: the gross revenue recognized for the period ended August 31, 2020 was $384,543.
We may not acquire market share or achieve profits due to competition in our industries.
Our Company operates in highly competitive marketplaces, such as the nicotine, pharmaceutical and CBD industries, respectively, with various competitors. Increased competition may result in reduced licensing rates and/or loss of market share, either of which would seriously harm its business and results of operations. Management cannot be certain that the Company will be able to compete against current or future competitors or that competitive pressure will not seriously harm its business. Some of our Company’s competitors are much larger and have greater access to capital, sales, marketing and other resources. These competitors may be able to respond more rapidly to new regulations or devote greater resources to the development and promotion of their business model than the Company can. Furthermore, some of these competitors may make acquisitions or establish co-operative relationships among themselves or with third-parties in the industry to increase their ability to rapidly gain market share.
| 8 |
| Table of Contents |
Without additional financing to develop our business plan, our business may fail.
Because we have generated only minimal revenue from our business and cannot anticipate when we will be able to generate meaningful revenue from our business, we will need to raise additional funds to conduct and grow our business. We do not currently have sufficient financial resources to completely fund the development of our business plan. We anticipate that we will need to raise further financing. We do not currently have any arrangements for financing and we can provide no assurance to investors that we will be able to find such financing if required. The most likely source of future funds presently available to us is through the sale of equity capital. Any sale of share capital will result in dilution to existing security-holders.
If we are unable to hire and retain key personnel, we may not be able to implement our business plan.
Our success is largely dependent on our ability to hire highly qualified personnel. This is particularly true in those parts of our business that are related to intellectual property generation or exploitation. These individuals are in high demand and we may not be able to attract the personnel we need. In addition, we may not be able to afford the high salaries and fees demanded by qualified personnel or may lose such employees after they are hired. Failure to hire key personnel when needed, or on acceptable terms, would have a significant negative effect on our business.
Our Company has a limited operating history and an evolving business model, which raises doubt about our ability to achieve profitability or obtain future financing.
Our Company has a minimal history of operations and our business model is still evolving and subject to change. Our revenues are dependant mostly upon licensing DehydraTECH and on those third-party licensees generating usage fees by successfully selling products utilizing DehydraTECH. Our licensees may also be subject to regulatory approval of their products that utilize DehydraTECH, which may not occur before they can bring their products to market and we generate usage licensing revenues from them.
Our Company’s ability to continue as a going concern is dependent upon our ability to obtain adequate financing and/or to reach profitable levels of operations. In that regard, we have no proven history of performance, earnings or success. There can be no assurance that we will achieve profitability or obtain future financing.
Our auditors have indicated doubt about our ability to continue as a going concern.
We have suffered recurring losses from operations. The continuation of our Company as a going concern is dependent upon our Company attaining and maintaining profitable operations and/or raising additional capital. Our financial statements do not include any adjustment relating to the recovery and classification of recorded asset amounts or the amount and classification of liabilities that might be necessary should our Company discontinue operations. The recurring losses from operations and net capital deficiency raise substantial doubt about the Company’s ability to continue as a going concern. Our independent registered public accounting firm issued a report dated October 14, 2020 in connection with the audit of our financial statements as of September 30, 2020, which included an explanatory paragraph describing the existence of conditions that raise substantial doubt about our ability to continue as a going concern. In addition, the notes to our financial statements for the year ended September 30, 2020, included in our Annual Report on Form 10-K filed with the Commission on October 15, 2020, contain a disclosure describing the existence of conditions that raise substantial doubt about our ability to continue as a going concern.
| 9 |
| Table of Contents |
A wide range of economic and market factors may negatively impact our operating results.
Our operating results will be affected by a wide variety of factors that could materially affect revenues and profitability, including the timing and cancellation of customer orders and projects, competitive pressures on pricing, availability of personnel, and market acceptance of our services. As a result, we may experience material fluctuations in future operating results on a quarterly and annual basis which could materially affect our business, financial condition and operating results.
Results of earlier studies may not be predictive of future results and planned or ongoing studies may not establish an adequate efficacy profile for DehydraTECH enabled products.
To date, DehydraTECH has been studied only with CBD, nicotine and, preclinically, with limited antiviral drugs as potential active pharmaceutical ingredients (“APIs”). The results of these studies and trials of DehydraTECH and future studies may not be predictive of the results of subsequent trials incorporating these or other APIs. Studies published to date on DehydraTECH have demonstrated positive results through oral and topical delivery of CBD and oral delivery of nicotine and the antiviral drug therapies Darunavir and Efavirenz, though in preclinical capacities. These results may not be replicated in subsequent, expanded studies or trials that incorporate the same or other API payloads, such as alternative therapies for COVID-19.
Licensees subject to significant regulatory requirements and testing protocols, such as those required by the US Food and Drug Administration (“FDA”), and comparable foreign regulators, must successfully complete multi-phase testing and the results of our studies may not be reflected in the outcome of the testing performed related to their products. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier studies, and we cannot be certain that our licensees will not face similar setbacks.
We rely on third-party manufacturers and suppliers and we intend to rely on third parties to produce preclinical and clinical supplies of active pharmaceutical ingredients, or APIs, including CBD.
We rely on third parties to supply the materials for, and manufacture, our research and development, preclinical and clinical trial APIs. We do not own manufacturing facilities or supply sources for such components and materials. There can be no assurance that our supply of research and development, preclinical and clinical development drugs and other materials will not be limited, interrupted, restricted in certain geographic regions or of satisfactory quality or continue to be available at acceptable prices. In particular, any replacement of our API manufacturer could require significant effort and expertise because there may be a limited number of qualified manufacturers.
The manufacturing process for the APIs we use, including CBD, are subject to FDA, DEA and other regulatory authority review. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards such as cGMP. In addition, our manufacturers must ensure therapeutic consistency among batches, including preclinical, clinical and, if approved, marketing batches. Demonstrating such consistency may require typical manufacturing controls as well as clinical data. Our manufacturers must also ensure that our batches conform to complex release specifications. Further, manufacturers of controlled substances must obtain and maintain necessary DEA and state registrations and registrations with applicable foreign regulatory authorities, and must establish and maintain processes to ensure compliance with DEA and state requirements and requirements of applicable foreign regulatory authorities governing, among other things, the storage, handling, security, recordkeeping and reporting for controlled substances. In the event that any of our suppliers or manufacturers fails to comply with such requirements or to perform its obligations to us in relation to quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may be forced to enter into an agreement with another third party, which we may not be able to do on reasonable terms, if at all. In some cases, the technical skills or technology required to manufacture the APIs may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills or technology to another third party and a feasible alternative may not exist. These factors would increase our reliance on such manufacturer or require us to obtain a license from such manufacturer in order to have another third party manufacture our clinical trial products. If we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop product candidates in a timely manner or within budget.
| 10 |
| Table of Contents |
We expect to continue to rely on third-party manufacturers. To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner consistent with contractual and regulatory requirements, including those related to quality control and assurance. If we are unable to obtain or maintain third-party manufacturing for product candidates, or to do so on commercially reasonable terms, we may not be able to develop and commercialize our Technology successfully. Our or a third party’s failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
|
| · | an inability to initiate or continue preclinical studies or clinical trials; |
|
|
|
|
|
| · | delay in submitting regulatory applications, or receiving regulatory approvals, for product candidates; |
|
|
|
|
|
| · | loss of the cooperation of a collaborator; |
|
|
|
|
|
| · | subjecting our Technology to additional inspections by regulatory authorities; and |
|
|
|
|
|
| · | in the event of approval to market and commercialize a product candidate, an inability to meet commercial demands. |
If our commercial partners fail to obtain FDA authorization to market their products that use our Technology as tobacco products, they will be unable to commercialize this potential product in the U.S.
There can be no assurance that any Premarket Tobacco Product Application (“PMTA”) submitted to FDA by our commercial partners utilizing our Technology will be authorized by the FDA. In addition, there can be no assurance that all necessary approvals will be granted for their potential tobacco products or that review or actions will not involve delays caused by requests for additional information or testing that could adversely affect the time and cost to market and sell their potential tobacco products.
The development, testing, manufacturing, and marketing of their potential tobacco products are subject to extensive regulation by governmental authorities in the United States. In particular, the process of obtaining authorization by the FDA is costly and time consuming, and the time required for such authorizations is uncertain. Any PMTA must undergo an extensive regulatory authorization process mandated by the FDA.
The FDA could force the removal of our products from the U.S. market.
The FDA has broad authority over the regulation of tobacco products. The FDA could, among other things, force our commercial partners to remove from the U.S. market their tobacco products even after the FDA authorization of any future PMTA, as well as the FDA could levy fines or change their regulations on advertising. Any adverse action by the FDA could have a material adverse impact on our business.
Any product candidates that receive regulatory approval will be subject to ongoing and continued regulatory review, which may result in significant expense and limit our ability to commercialize such products.
For any product candidates containing controlled substances, we and our commercial partners will be subject to ongoing DEA regulatory obligations, including, among other things, annual registration renewal, security, recordkeeping, theft and loss reporting, periodic inspection and annual quota allotments for the raw material for commercial production of our products. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP and other regulations. If we, our commercial partners or the manufacturing facilities for product candidates using our Technology fail to comply with applicable regulatory requirements, a regulatory agency may:
| 11 |
| Table of Contents |
|
| · | suspend any ongoing clinical trials or delay or prevent the initiation of clinical trials; |
|
|
|
|
|
| · | delay or refuse to approve pending applications or supplements to approved applications that have been filed; |
|
|
|
|
|
| · | refuse to permit drugs or to be imported or exported to or from the United States; |
|
|
|
|
|
| · | seize or detain drugs or require a recall; |
|
|
|
|
|
| · | suspend or impose restrictions or additional requirements on operations, including costly new manufacturing quality requirements; |
|
|
|
|
|
| · | commence criminal investigations and prosecutions; |
|
|
|
|
|
| · | impose restrictions on the marketing or manufacturing of a product, suspend or withdraw product approvals or revoke necessary licenses; and |
|
|
|
|
|
| · | issue warning letters, show cause notices or untitled letters describing alleged violations, which may be publicly available. |
The FDA's regulations, policies or guidance may change and new or additional statutes or government regulations may be enacted that could prevent or delay regulatory approval of our product candidates or further restrict or regulate post-approval activities. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from pending or future legislation or administrative action. If we or are commercial partners are not able to achieve and maintain regulatory compliance, drugs using the Technology may not be marketed, which would adversely affect our ability to generate revenue and achieve or maintain profitability.
Intellectual property and technology development involves a lengthy and expensive process, with an uncertain outcome. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, all of the research and development for all industry segments.
We may experience delays in initiating or completing our planned studies or trials in the future, and we may experience numerous unforeseen events during, or as a result of, any future studies or trials that we conduct that could delay or prevent our ability to conduct the research, including:
|
| · | regulators or institutional review boards (“IRBs”), or ethics committees may not authorize us or our investigators to commence a study or trial at a prospective trial site and/or additional governmental regulatory authority authorizations may be required from time-to-time to do so for which there is no assurance that we will be able to satisfy their approval conditions in a timely fashion if at all, whether due to financial or other unforeseen constraints; |
|
|
|
|
|
| · | we may experience delays in reaching, or fail to reach, agreement on acceptable terms with prospective trial sites and prospective contract research organizations (“CROs”), the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
|
|
|
|
|
| · | we may experience delays in recruiting, or be unable to recruit, a sufficient number of suitable participants to participate in our studies or trials; |
|
|
|
|
|
| · | the participants and sites who participate in our studies or trials may not comply with required protocols rendering the results insufficient or uninterpretable; |
| 12 |
| Table of Contents |
|
| · | studies or trials of various APIs may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional studies or trials or we may decide to abandon development programs related to those APIs; |
|
|
|
|
|
| · | the number of participants required for studies or trials of an API may be larger than we anticipate, enrollment in these studies or trials may be slower than we anticipate or participants may drop out or fail to return for follow-up at a higher rate than we anticipate; |
|
|
|
|
|
| · | our third party contractors may fail to comply with regulatory or legal requirements or meet their contractual obligations to us in a timely manner, or at all, or may deviate from the protocol or drop out, which may require that we find new contractors to perform the work; |
|
|
|
|
|
| · | we may elect to, or regulators or IRBs or ethics committees may require that we or our investigators, suspend or terminate our research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; |
|
|
|
|
|
| · | the cost of studies or trials of an API may be greater than we anticipate; |
|
|
|
|
|
| · | any changes in regulatory requirements and guidance that require amending or submitting new protocols; |
|
|
|
|
|
| · | regulators may require us to submit additional data or impose other requirements before permitting us to initiate a study or trial. |
We could encounter delays if a study or trial is suspended or terminated by us or by the IRBs of the institutions in which they are being conducted. Such authorities may impose such a suspension or termination due to a number of factors, including changes in governmental regulations or administrative actions or lack of adequate funding to continue the study or trial. Further, the IRB may disagree with our design or may change the requirements for approval even after it has reviewed and commented on the design.
Our research and development costs will also increase if we experience delays in testing or regulatory approvals. We do not know whether any of our studies or trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Any delays in our development programs may significantly harm our business, prospects, financial condition and results of operations.
The longer-term growth of our business depends on our ability to expand our portfolio of patents and industry segments where DehydraTech is demonstrably applicable, which may require substantial financial resources and may ultimately be unsuccessful.
The longer-term growth of our business depends upon our ability to expand our patent portfolio of applicable APIs and molecules and delivery methods. We may also be required to evidence that DehydraTECH’s demonstrated efficacy also works with other APIs and molecules prior to acceptance and adoption within those segments. The required research and development programs required to develop the evidence may require substantial financial resources and may ultimately be unsuccessful.
Loss of consumer confidence in our Company or in our industry may harm our business.
Demand for our services may be adversely affected if consumers lose confidence in the quality of our services or the industry’s practices. Adverse publicity may discourage businesses from buying our services and could have a material adverse effect on our financial condition and results of operations.
| 13 |
| Table of Contents |
Conflicts of interest between our Company and our independent directors and executive management may result in a loss of business opportunity.
Our independent directors and members of our executive management are not obligated to exclusively commit their time and attention to our business and, accordingly, they may encounter a conflict of interest in allocating their time between our future operations and those of other businesses. In the course of their other business activities, they may become aware of investment and business opportunities which may be appropriate for presentation to us as well as other entities to which they owe a fiduciary duty. As a result, they may have conflicts of interest in determining to which entity a particular business opportunity should be presented. They may also in the future become affiliated with entities, engaged in business activities similar to those we intend to conduct.
In general, officers and directors of a corporation are required to present business opportunities to a corporation if:
|
| · | The corporation could financially undertake the opportunity; |
|
|
|
|
|
| · | The opportunity is within the corporation’s line of business; and |
|
|
|
|
|
| · | It would be unfair to the corporation and its stockholders not to bring the opportunity to the attention of the corporation. |
We have adopted a code of ethics that obligates our directors, officers and employees to disclose potential conflicts of interest and prohibits those persons from engaging in such transactions without our consent. Despite our intentions, conflicts of interest may nevertheless arise which may deprive our Company of a business opportunity, which may impede the successful development of our business and negatively impact the value of an investment in our Company.
We could be required to enter into fixed price contracts which will expose us to significant market risk.
Fixed price contracts require the service provider to perform all agreed services for a specified lump-sum amount. We anticipate a material percentage of our services will be performed on a fixed price basis. Fixed price contracts expose us to some significant risks, including under-estimation of costs, ambiguities in specifications, unforeseen costs or difficulties, and delays beyond our control. These risks could lead to losses on contracts which may be substantial, and which could adversely affect the results of our operations.
We may not be able to obtain all of the licenses necessary to operate our business, which would cause our business to fail.
Our operations may require licenses and permits from various governmental authorities to conduct our business activities. We believe that we will be able to obtain all necessary licenses and permits under applicable laws and regulations for our operations and believe we will be able to comply in all material respects with the terms of such licenses and permits. However, such licenses and permits are subject to change in various circumstances. There can be no guarantee that we will be able to obtain or maintain all necessary licenses and permits.
If we fail to effectively manage our growth, our future business results could be harmed and our managerial and operational resources may be strained.
As we proceed with our business plan, we expect to experience significant and rapid growth in the scope and complexity of our business. We will need to add staff to market our services, manage operations, handle sales and marketing efforts and perform finance and accounting functions. We will be required to hire a broad range of additional personnel in order to successfully advance our operations. This growth is likely to place a strain on our management and operational resources. The failure to develop and implement effective systems, or to hire and retain sufficient personnel for the performance of all of the functions necessary to effectively service and manage our potential business, or the failure to manage growth effectively, could have a materially adverse effect on our business and financial condition.
| 14 |
| Table of Contents |
The COVID-19 pandemic may have a negative impact on our business.
The emergence of COVID-19 around the world presents significant and unforecastable risk to the Company and its business plan. Restrictions on national and international travel, and required business closures, have made it increasingly difficult to carry out normal business activities related to corporate finance efforts, to the pursuit of new customers for the Company’s products and services, and to retail customers throughout North America who might otherwise access the products of the Company’s business-to-business partners. As a result, the COVID-19 pandemic will almost certainly increase risks of lower revenues and higher losses for the Company. We are monitoring our licensees and are working with them, where possible, to prevent default and contract terminations. In some cases, we, in accordance with the relevant governing agreements, have issued termination of contract notices to some of our licensees. These terminations resulted in $50,000 in write offs of accounts receivable.
As a result of COVID-19, the Company is encountering significant challenges in executing its business plan and normal business operations and does not have sufficient resources to withstand a protracted term during which most business activities are curtailed. In addition, we have implemented cost containment initiatives to reduce operating expenses and preserve cash such as dismissal of one employee, termination of contracts with two consultants and reduction of compensation payable to certain other consultants. We may need to dismiss additional employees or terminate services contracts in order to preserve resources. We have not had to close operations or locations as our contractors and staff can work remotely and our third-party fulfillment centers continue to operate.
The Company may not be able to monetize any opportunities related to the COVID-19 outbreak.
The Company is currently investigating whether there may be any emerging opportunities resulting from the COVID-19 crisis for its patented DehydraTECH technology that has been tested for its delivery of other compounds and drugs including CBD and nicotine, and whether the apparent benefits of DehydraTECH, namely increased bioavailability, enhanced intestinal tolerability and biliary functionality might be applicable to compounds or drugs used to treat symptoms caused by COVID-19. This investigation is in the very early stages with an initial animal study (as noted below) having been completed with demonstrated improvements in antiviral drug delivery. On March 19, 2020, the Company announced that it intends to conduct a pilot human pharmacokinetic exploratory study in healthy volunteers of three antiviral drugs that have previously been studied against other coronavirus strains, comparing DehydraTECH formulations to controls without DehydraTECH enhancement. Subsequent to March 19, 2020, the Internal Review Board (“IRB”) of one of the universities advised us to limit the study to two of the original antiviral drugs. Based on the requirements of the IRB we have modified the study to two antiviral drugs. We intend to conduct the study at a leading Canadian university to which we have already submitted a study design and plan and from which we have already received ethics board approval. The study, however, remains subject to further government regulatory approval. The Company is currently in the process of pursuing the necessary steps to file for study approval from Canadian federal regulators.
In parallel, the Company launched a separate rodent antiviral study to evaluate pharmacokinetic benefits from the use of DehydraTECH in the delivery of representative drugs from two classes of antiviral drugs under investigation for treatment of COVID-19. The results of that animal study were released on December 1, 2020 whereby the DehydraTECH enhanced antiviral drug formulations demonstrated increased delivery of the antiviral drugs into the bloodstream of the animals. The results of this animal study have encouraged the Company to conduct expanded investigations into antiviral drug delivery enhancement, with such investigations including remdesivir (a nucleotide reverse transcriptase inhibitor); as well as three additional drugs known to target the main protease associated with SARS-CoV-2 infection. The Company intends to make its research results available to researchers throughout the world looking to maximize the delivery of their own drug investigations. The Company’s business model relies on performing early stage studies like these to help support its efforts to form commercial relationships with more established companies.
The Company continues to monitor governmental programs being released to assist with the COVID-19 pandemic.
| 15 |
| Table of Contents |
DehydraTECH has never been approved for the treatment of disease.
In order for a licensee to commercialize a product that utilizes DehydraTECH for the treatment of any disease, they must obtain regulatory approvals for their product of such treatment for that indication. Satisfying regulatory requirements is an expensive process that typically takes many years and involves compliance with requirements covering research and development, testing, manufacturing, quality control, labeling, and promotion of drugs for human use. To obtain necessary regulatory approvals, a licensee must, among other requirements, complete clinical trials demonstrating that their product is safe and effective for a particular indication. There can be no assurance that their product enhanced by DehydraTECH will prove to be safe and effective, that the clinical trials will demonstrate the necessary safety and effectiveness of the product candidates, or that a licensee will succeed in obtaining regulatory approval for any treatment developed even if such safety and effectiveness are demonstrated.
Any delays or difficulties encountered in such clinical trials may delay or preclude regulatory approval from the FDA or from international regulatory organizations. Any delay or preclusion of regulatory approval would be expected to delay or preclude the commercialization of their product that utilizes DehydraTECH. Examples of delays or difficulties that may be encountered during clinical trials include without limitation the following:
|
| · | Clinical trials may not yield sufficiently conclusive results for regulatory agencies to approve the use of DehydraTECH; |
|
|
|
|
|
| · | DehydraTECH enhanced formulations may fail to be more effective than current therapies, or to be effective at all; |
|
|
|
|
|
| · | DehydraTECH enhanced formulations may have adverse side effects, which could cause them to be delayed or precluded from receiving regulatory approval or otherwise expose us to significant commercial and legal risks; |
|
|
|
|
|
| · | It may take longer than expected to determine whether or not a treatment is effective; |
|
|
|
|
|
| · | Patients involved in the clinical trials may suffer severe adverse side effects even up to death, whether as a result of treatment with DehydraTECH enhanced formulations, the withholding of such treatment, or other reasons (whether within or outside of our control); |
|
|
|
|
|
| · | Failure to be able to enroll a sufficient number of patients in the clinical trials; |
|
|
|
|
|
| · | Patients enrolled in the clinical trials may not have the characteristics necessary to obtain regulatory approval for a particular indication or patient population; |
|
|
|
|
|
| · | Inability to produce sufficient quantities of DehydraTECH enhanced formulations to complete the clinical trials; |
|
|
|
|
|
| · | Failure to obtain and/or maintain, any required governmental approvals; |
|
|
|
|
|
| · | If approval for commercialization is granted, it is possible the authorized use will be more limited than is necessary for commercial success, or that approval may be conditioned on completion of further clinical trials or other activities, which will cause a substantial increase in costs; |
|
|
|
|
|
| · | If granted, approval may be withdrawn or limited if problems with DehydraTECH enhanced formulations emerge or are suggested by the data arising from their use or if there is a change in law or regulation. |
Any success achieved at a given stage of the clinical trials does not guarantee that the future achievement of success at any subsequent stage, including without limitation, final FDA approval.
| 16 |
| Table of Contents |
Delays or rejections in the regulatory approval process because of additional government regulation resulting from future legislation or administrative action, or from changes in the policies of the FDA or other regulatory bodies during the period of product development, clinical trials, or regulatory review may occur. Failure to comply with applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production, or an injunction preventing certain activity, as well as other regulatory action against our product candidates or us.
Our success is dependent on our licensees’ ability to successfully navigate the risks and obstacles associated with obtaining FDA approval for any DehydraTECH enhanced formulation of their product.
We have relied, and will rely in the future, on third parties to conduct our studies and trials. If these third parties do not appropriately carry out their contractual obligations, fail to conduct high-quality trials in compliance with regulations or meet expected deadlines, our research programs may be delayed or could fail to develop required data.
We do not have the ability to conduct our studies or trials independently. We have and will continue to rely on third parties, including third-party facilities, participants and consultants, to monitor, manage data for, participate in and execute our ongoing planned research protocols. Any failure of these third parties to meet their contractual or regulatory obligations may have an adverse effect on the results of our studies or trials.
The third parties conducting our studies or trials on our behalf are not our employees, and except for remedies available to us under our agreements with such contractors, we cannot control whether or not they devote sufficient time, skill and resources to our programs. To the extent we are unable to identify and successfully manage the performance of third-party service providers in the future, our business may be adversely affected. If these third parties do not successfully carry out their contractual obligations or meet expected deadlines, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our protocols, regulatory requirements or for other reasons, our studies or trials may be extended, delayed or terminated and we may not be able to obtain, or may be delayed in obtaining, appropriate research data. As a result, our results of operations could be harmed, our costs could increase and our ability to generate revenues could be delayed or impaired for the API or molecule under research.
Any change to government regulation/administrative practices may have a negative impact on our ability to operate and our profitability.
The laws, regulations, policies or administrative practices of any government body, organization or regulatory agency in the United States, Canada, or any other jurisdiction may be changed, applied or interpreted in a manner which will fundamentally alter the ability of our Company to carry on our business.
The actions, policies or regulations, or changes thereto, of any government body or regulatory agency, or other special interest groups, may have a detrimental effect on us. Any or all of these situations may have a negative impact on our ability to operate and/or our profitably.
The majority of our directors and officers are residents of other countries other than the United States and, as a result, investors may find it difficult to enforce, within the United States, any judgments obtained against our company or our directors and officers.
Our head office and the majority of our assets are located in Kelowna, British Columbia and we rent administrative office space in Phoenix, Arizona. In addition, a majority of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons’ assets are located outside the United States. As a result, it may be difficult for investors to enforce within the United States any judgments obtained against our Company or our officers or directors, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof.
| 17 |
| Table of Contents |
Risks Associated with our Intellectual Property
Our failure to protect our intellectual property may have a material adverse effect on our ability to develop and license DehydraTECH.
Because patents involve complex legal and factual questions, the issuance, scope, validity, and enforceability of patents cannot be predicted with certainty.
Some of our patent pending applications may not be granted as patents. Even if patents are issued, they may not be issued with claims of sufficient breadth to protect DehydraTECH or may not provide us with competitive advantage against competitors with similar products or technologies. Issued patents may be challenged, invalidated, or circumvented. If patents issued to us are invalidated or found to be unenforceable, we could lose the ability to exclude others from making, using or selling the inventions claimed. Moreover, an issued patent does not give us the right to use the patented technology or commercialize a product using the technology. Third-parties may have blocking patents that could be used to prevent us from developing our products, selling our products, or commercializing DehydraTECH. Others may also independently develop products or technologies similar to those that we have developed or may reverse engineer or discover our trade secrets through proper means.
Enforcing a claim that a third party infringes on, has illegally obtained or is using an intellectual property right, is expensive and time-consuming and the outcome is unpredictable. In addition, enforcing such a claim could divert management’s attention from our business. If any intellectual property rights were to be infringed, disclosed to, or independently developed by a competitor, our competitive position could be harmed. Any adverse outcome of such litigation or settlement of such dispute could subject us to significant liabilities and could put one or more of our patent pending applications at risk of being invalidated.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is risk that some of our confidential information could be compromised. This disclosure could provide our competitors with access to our proprietary information and may harm our competitive position.
If we are unable to obtain and maintain sufficient patent protection, or if the scope of the patent protection is not sufficiently broad, our competitors could develop technology similar to ours.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our intellectual property. If we do not adequately protect or enforce our intellectual property, competitors may be able to erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. To protect our intellectual property, we file patent applications in the United States and abroad. The patent application and examination process is expensive, complex and time-consuming. We may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner.
| 18 |
| Table of Contents |
We may become involved in lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive, time consuming and unsuccessful.
Competitors and other third parties may infringe, misappropriate or otherwise violate our patents and other intellectual property rights. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming and divert the time and attention of our management, business and scientific personnel. In addition, many of our adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we can.
A court may disagree with our allegations and may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the third-party technology in question. Furthermore, the other party could counterclaim that we infringe their intellectual property or counterclaim that a patent we have asserted against them is invalid or unenforceable, or both. In patent litigation in the United States, counterclaims challenging the validity, enforceability or scope of asserted patents are commonplace. Similarly, third parties may initiate legal proceedings against us seeking a declaration that certain of our intellectual property rights are non-infringed, invalid, or unenforceable. The outcome of any such proceeding is generally unpredictable.
We may not be able to effectively enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending patents in all countries throughout the world would be prohibitively expensive. Our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, the patent laws of some foreign countries do not provide protection to the same extent as the laws of the United States. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our intellectual property rights. Legal actions to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and resources from other aspects of our business. While we intend to protect our intellectual property, we do not expect that we will be able to initiate or maintain legal efforts in all jurisdictions.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The United States Patent and Trademark Office (“USPTO”) and various foreign governmental patent agencies require compliance with their procedural, documentary, fee payment and other provisions during the patent application process. Periodic maintenance fees on issued patents often must be paid to the USPTO and foreign patent agencies over the lifetime of each patent. While an unintentional lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications covering our intellectual property, we may not be able to stop a competitor from utilizing our Technology, which would have a material adverse effect on our business.
| 19 |
| Table of Contents |
Risks Related to CPG/Oral Products
Even if we develop food, consumer packaged goods (“CPG”) or intellectual property-based products or revenue streams, the potential profitability of each depends upon factors beyond the control of the Company.
The potential profitability of food and CPG products and of intellectual property revenue streams is dependent upon many factors beyond our control. For instance, prices and markets for food products are unpredictable, highly volatile, potentially subject to controls or any combination or other factors, and respond to changes in domestic, international, political, social and economic environments. These changes and events may materially affect our future financial performance. These factors cannot be accurately predicted and the combination of these factors may result in our Company not receiving an adequate return on invested capital.
In addition, a product or technology that is initially successful and even profitable may not remain so due to changes in consumer demand, regulatory environments, or other causes. There is no assurance that an initially successful product or technology will remain so.
Food, CPG and hemp products are subject to regulation which may cause substantial delays or require capital outlays in excess of those anticipated causing an adverse effect on our company.
Food, CPG and hemp production, marketing, sales and safety operations, are subject to federal, state, and local laws relating to the protection of human health and safety. Food production and hemp operations are each also subject to federal, state, and local laws and regulations which seek to maintain health and safety standards through a wide variety of regulations. Various permits from government bodies may be required by us in order to conduct our business. Regulations and standards imposed by federal, provincial, or local authorities may be changed at any moment in time and any such changes may have material adverse effects on our activities. Changes in regulations are impossible to foresee and could be disruptive or destructive to our business plans and execution. Moreover, compliance with such laws may cause substantial delays or require capital outlays in excess of those anticipated, thus causing an adverse effect on us. Additionally, we may be subject to liability for contaminants or other damages. To date, we have not been required to spend any material amount of money or resources on compliance with environmental regulations. However, we may be required to do so in the future and this may affect our ability to expand or maintain our operations.
Uncertain demand for our products or technology may cause our business plan to be unprofitable.
Demand for oral products, CPG, technology delivery benefits and hemp related products is dependent on a number of social, political and economic factors that are beyond the control of our Company. While we believe that demand for these products will continue to grow across North America, there is no assurance that such increase in demand will happen or that our endeavors will be profitable.
The failure to secure customers may cause our operations to fail.
We currently have very few customers. We will require new customers and/or business partners in order to sustain our operations and attempt to achieve profitability.
There can be no assurance that we will develop any product that will meet with widespread consumer acceptance.
Often, both new and established oral product and CPG product companies fail to generate consumer interest on a regular basis. There is no assurance that any oral product or CPG product that we market will be successfully adopted by consumers or will still be in demand at a future time. If we cannot develop and sell products in commercial quantities, our business could fail.
| 20 |
| Table of Contents |
The oral product and CPG industries are highly competitive and there is no assurance that we will be successful in developing or successfully selling products.
The oral product and CPG industries are intensely competitive. We compete with numerous entities, including many oral product manufacturing and production companies, which have substantially greater technical, financial and operational resources and staff. Accordingly, there is a high degree of competition for desirable distribution channels, “shelf space” and salespeople in both the oral product and CPG industries. We cannot predict if the necessary funds can be raised to assist in our development of any distribution channels that may be helpful to our ability to generate sales and potential profits.
The marketability of oral products and CPG products will be affected by numerous factors beyond our control which may result in us not receiving an adequate return on invested capital to be profitable or viable.
The marketability of oral products and CPG products will be affected by numerous factors beyond our control. These factors include market fluctuations in consumer preferences for various oral product items based on factors such as pricing, macro trends for certain ingredients or flavors, ruling by regulators on health issues associated with certain foods, and more. The exact effect of these factors cannot be accurately predicted, but the combination of these factors may result in us not receiving an adequate return on invested capital to be profitable or viable.
We are not the “operator” of vertically integrated oral product production facilities, and so we are exposed to the risks of our third-party operators.
We rely on the expertise of contracted third-parties for their judgment, experience and advice related to the manufacturing and/or packaging of oral products. We can give no assurance that these third-party operators or consultants will always act in our best interests, and we are exposed to their operations and actions and advice in those operations and activities in which we are contractually bound.
Our management has limited experience and training in the oral product processing and manufacturing industries, and in the hemp products industries, and could make decisions that negatively impact our licensee operations and our Company.
Because our management has limited experience and training in the oral product processing and manufacturing industry, and in the hemp products industry, we may not have sufficient expertise to make best practices decisions regarding our operations and/or those of our corporate licensees. It is possible that, due to our limited knowledge, we might elect to undergo manufacturing processes and incur financial burdens that a more experienced oral product manufacturing team might elect not to complete. Our ability to internally evaluate food and hemp operations and opportunities could be less thorough than that of a more highly trained management team.
The Farm Bill, FDA policies and other regulations materially affect our CBD products and Licensees
In conjunction with the enactment of the Agriculture Improvement Act of 2018 (the “Farm Bill”), the FDA released a statement about the status of CBD as a nutritional supplement, and the agency’s actions in the short term with regards to CBD will guide the industry. We will strive to comply with all guidelines and regulations as they evolve. The regulation of CBD products is currently in constant flux and any difficulties in compliance with future government regulation could increase our operating costs and adversely impact our results of operations in future periods. Furthermore, violations of these laws, or alleged violations, could disrupt our business or the business of our licensees and result in a material adverse effect on our operations. In addition, we cannot predict the nature of any future laws, regulations, interpretations or applications, and it is possible that regulations may be enacted in the future that will be directly applicable to our business.
| 21 |
| Table of Contents |
We do not currently believe that we are required to seek FDA approval for DehydraTECH independent of any drug, CPG, oral product, or other product in the industries in which are involved, and as such we do not plan to seek FDA approval. If regulation evolves such that we are required to seek approval, we will endeavor to do so. This may require us to incur substantial costs associated with legal and compliance fees and adversely affect our results of operations.
Possible yet unanticipated changes in federal and state law could cause products containing hemp-derived CBD oil to be illegal, or could otherwise prohibit, limit or restrict the commercialization of any Technology-enhanced products containing CBD.
While we are currently winding down our distribution of Lexaria branded products containing hemp-derived CBD, we have licensees who produce and distribute hemp-derived CBD products that are enhanced with DehydraTECH.
The Farm Bill delegates the authority to the states to regulate and limit the production of hemp and hemp-derived products within their territories. Although many states have adopted laws and regulations that allow for the production and sale of hemp and hemp-derived products under certain circumstances, no assurance can be given that such state laws may not be repealed or amended such that our intended products containing hemp-derived CBD would once again be deemed illegal under the laws of one or more states now permitting such products, which in turn would render such intended products illegal in those states under federal law even if the federal law is unchanged. In the event of either repeal of federal or of state laws and regulations, or of amendments thereto that are adverse to our or our licensee’s products, we may be adversely impacted with respect to CBD product revenue or royalties.
Sources of hemp-derived CBD depend upon legality of cultivation, processing, marketing and sales of products derived from those plants under state law.
Hemp-derived CBD can only be legally produced in states that have laws and regulations that allow for such production and that comply with the Farm Bill. We purchase all of our hemp-derived CBD from licensed growers and processors in states where such production is legal. As described in the immediately above risk factor, possible yet unanticipated changes in federal and state law could cause any of our current products that we are ending production of and which contain hemp-derived CBD oil, to be illegal or could otherwise prohibit, limit or restrict any of our products containing CBD in the event of repeal or amendment of laws and regulations which are now favorable to the hemp industry in such states, we would be required to locate new suppliers in states with laws and regulations that qualify under the Farm Bill. If we were to be unsuccessful in arranging new sources of supply of our raw ingredients, or if our raw ingredients were to become legally unavailable, our intended business plan with respect to such products could be adversely impacted.
Because our distributors may only sell and ship our products containing hemp-derived CBD in states that have adopted laws and regulations qualifying under the Farm Bill, a reduction in the number of states having such qualifying laws and regulations could limit, restrict or otherwise preclude the sale of intended products containing hemp-derived CBD.
The interstate shipment of hemp-derived CBD from one state to another is legal only where both states have laws and regulations that allow for the production and sale of such products and that qualify under the Farm Bill. Therefore, the marketing and sale of our intended products containing hemp-derived CBD is limited by such factors and is restricted to such states. Although we believe we may lawfully sell any of our finished products, including those containing CBD, in a majority of states, a repeal or adverse amendment of laws and regulations that are now favorable to the distribution, marketing and sale of finished products we intend to sell could significantly limit, restrict or prevent us from generating revenue related to our products that contain hemp-derived CBD. Any such repeal or adverse amendment of now favorable laws and regulations could have an adverse impact on our business plan with respect to such products.
| 22 |
| Table of Contents |
Due to recent expansion into the CBD, nicotine and pharmaceutical industries, we may have a difficult time obtaining the various insurances that are desired to operate our business, which may expose us to additional risk and financial liability.
Insurance that is otherwise readily available, such as general liability, and directors and officer’s insurance, may become more difficult for us to find, and more expensive, due to our sale of products containing hemp-derived CBD (which we are discontinuing), our research into alternative nicotine delivery methods and enhanced delivery of pharmaceutical compounds. There are no guarantees that we will be able to find such insurances in the future, or that the cost will be affordable to us. If we are forced to go without such insurances, it may prevent us from entering into certain business sectors, may inhibit our growth, and may expose us to additional risk and financial liabilities.
Changing consumer preferences may cause DehydraTECH enhanced products to be unsuccessful in the marketplace.
The decision of a potential client to purchase DehydraTECH enhanced products may be motivated by cultural phenomena or by perceived health or nutritional benefits. For example, the cultural desirability or popularity of hemp related products is subject to change due to factors beyond our immediate control. Similarly, the perceived nutritional or health related benefits of our products are subject to change in light of continuing research or the introduction of competitive products. Changes in consumer and commercial preferences, or trends, toward or away from cannabis or hemp related products would have a corresponding impact on the development of the market for our current and planned products. There can be no assurance that any DehydraTECH enhanced products will be successful in establishing or maintaining a significant share of the consumer market.
General economic factors may negatively impact the market for our planned products.
The willingness of businesses to spend time and money on non-essential oral product and health products may be dependent upon general economic conditions; and any material downturn may reduce the likelihood of consumers incurring costs toward what some may consider a discretionary expense item. Willingness by customers to buy our products may be dependent upon general economic conditions and any material downturn may reduce the potential profitability of the oral product sciences or medical marijuana business sectors.
If we fail to effectively and efficiently advertise, the growth of our business may be compromised.
The future growth and profitability of our oral products and CPG products business and our DehydraTECH licensing business will be dependent in part on the effectiveness and efficiency of our advertising and promotional expenditures, including our ability to (i) create greater awareness of our services, (ii) determine the appropriate creative message and media mix for future advertising expenditures, and (iii) effectively manage advertising and promotional costs in order to maintain acceptable operating margins. There can be no assurance that we will experience benefits from advertising and promotional expenditures in the future. In addition, no assurance can be given that our planned advertising and promotional expenditures will result in increased revenues, will generate levels of service and name awareness or that we will be able to manage such advertising and promotional expenditures on a cost-effective basis.
Risks Associated with our Historical Business Practices
The Company is a research and development company that has expended its efforts on improving and licensing DehydraTECH to third parties for use in their commercial consumer products. Historically, licensees of DehydraTECH have used DehydraTECH to enhance their cannabis related products. In order to evidence the effectiveness of DehydraTECH for the purposes of obtaining new licensees, the Company has directly produced certain consumer products containing hemp-derived CBD and containing less than 0.29% THC. The production and distribution of these products is not a business focus of the Company and the Company is currently winding down the production and distribution of such DehydraTECH enhanced hemp-derived CBD products. In addition, the Company, via its subsidiary CanPharm, has recently disposed of its ability to use or sublicense DehydraTECH with consumer products containing 0.3% or greater of THC, except in very limited circumstances and only outside of the United States and Canada . While the business focus of the Company is moving away from cannabis and CBD related products, and moving towards the use of DehydraTECH for the purposes of oral nicotine and pharmaceutical product development, the Company may be subject to regulatory liability for its past business practices with respect to the licensing of DehydraTECH to licensees who produce consumer products containing greater than 0.3% THC and/or for its historical production and distribution of CBD consumer products and the continued sale of outstanding inventory of CBD consumer products, which have not received FDA approval. We cannot predict whether any regulatory body may make a claim against the Company and if so whether the Company will have the financial resources to pay any imposed fines and/or to defend itself against such claim. Below are some of the risk factors attributed to our historical business practices.
| 23 |
| Table of Contents |
Unethical business practices of licensees who use DehydraTECH may compromise the growth and development of our business.
The production and sale of medical marijuana is an emerging industry in which business practices are not yet standardized and are subject to frequent scrutiny and evaluation by federal, state, provincial, and municipal authorities, academics, and media outlets, among others. Although we developed our historical business in accordance with best ethical practices, we may suffer negative publicity if we, our partners, contractors, or customers are found to have engaged in any environmentally, insensitive practices or other business practices that are viewed as unethical.
Because cannabis is a controlled substance in some regulatory jurisdictions, our former third-party licensee’s operations may be subject to regulatory actions.
The Company and its subsidiaries are not involved directly or indirectly in the cultivation, processing, distribution, or utilization of cannabis or cannabis derived components. All of the Company’s historically produced consumer products utilized legally sourced hemp and hemp components in their production. The Company has historically licensed its intellectual property to licensees that may utilize DehydraTECH in the production of products that contain contents which are locally or state approved but federally controlled. Where licensee’s products contain controlled contents any revenue streams from such licensee’s may be interrupted by regulatory involvement in their business.
The Company has no knowledge of any non-compliance by any of its licensees with the regulatory framework(s) in which its licensee(s) operate.
Cannabis remains illegal under U.S. federal law, and any change in the enforcement priorities of the federal government could negatively impact our current business operations.
We do not currently, nor at any time in our corporate history have we ever cultivated, grown, processed, manufactured or sold marijuana in any location. Although we believe this fact to provide protection against prosecution under marijuana legislation, we cannot provide any assurance to that effect. Historically, we have licensed DehydraTECH to entities who used DehydraTECH in connection with the production of products containing THC. All though these licensees had valid licenses in various North American jurisdictions to sell cannabis related products, we cannot guarantee that those historical business practices will not result in enforcement against the Company in the future.
The United States federal government regulates drugs through the Controlled Substances Act (the “CSA”), which places controlled substances, including cannabis, on one of five schedules. Cannabis is currently classified as a Schedule I controlled substance, which is viewed as having a high potential for abuse and having no currently accepted medical use in treatment in the United States. No prescriptions may be written for Schedule I substances, and such substances are subject to production quotas imposed by the United States Drug Enforcement Administration (the “DEA”). Because of this, doctors may not prescribe cannabis for medical use under federal law, although they can recommend its use under the First Amendment.
| 24 |
| Table of Contents |
Over 38 US States, including our state of incorporation, Nevada, have approved and regulate medical marijuana use. Similarly, eleven states and Washington D.C. have approved and regulate non-medical marijuana use by adults. Because cannabis is a Schedule I controlled substance, however, the development of a legal cannabis industry under the laws of these states is in conflict with the CSA, which makes cannabis use and possession illegal on a national level. The United States Supreme Court has confirmed that the federal government has the right to regulate and criminalize cannabis, including for medical purposes, and that federal law criminalizing the use of cannabis pre-empts state laws that legalize its use.
While we do not harvest, distribute, sell cannabis, or cannabis derived products, we may be irreparably harmed by the enforcement policies of the federal government. As of the date of this prospectus, we have disposed of our license to utilize DehydraTECH with cannabis that contains 0.3% or greater THC, however, pursuant to our historical business, and the license of our Technology to licensees in the U.S. cannabis industry , there is a potent ial that these historical business practices could be deemed to be aiding and abetting illegal activities, a violation of federal law.
Risks Associated with Our Common Stock
There has been no consistent active trading market for our common stock, and public trading of our common stock may continue to fluctuate substantially.
There has never been a consistent active trading market for our common stock. Our common stock is currently quoted on the OTCQX under the symbol “LXRP” and trades on the CSE under the symbol “LXX”. We plan on listing our common stock and warrants on Nasdaq under the symbols “LEXX” and “LEXXW”, respectively. There is no assurance that the trading market for our common stock and warrants will become more active or liquid. Furthermore, there can be no assurance any market maker will be interested in trading our stock. Therefore, it may be difficult to sell your shares of common stock or warrants if you desire or need to sell them. Our underwriters are not obligated to make a market in our securities, and even if they make a market, they can discontinue market making at any time without notice. Neither we nor the underwriters can provide any assurance that an active and liquid trading market in our securities will develop or, if developed, that such market will continue.
Moreover, the trading price of our common stock has fluctuated substantially over the past few years, and there remains a significant risk that our common stock price may continue to fluctuate substantially in the future in response to various factors, including any material variations in our periodic operating results, departures or additions of management or other key personnel, announcements of acquisitions, mergers, share consolidations, or new technology or patents, new product developments, significant litigation matters, gain or loss of significant licensees, significant capital transactions, substantial sales of our common stock in our trading market, and general and specific market and economic conditions.
Our stock price may be volatile, which could result in substantial losses to investors and litigation.
In addition to changes to market prices based on our results of operations and the factors discussed elsewhere in this “Risk Factors” section, the market price of and trading volume for our common stock may change for a variety of other reasons, not necessarily related to our actual operating performance. The capital markets have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock. In addition, the average daily trading volume of the securities of small companies can be very low, which may contribute to future volatility. Factors that could cause the market price of our common stock to fluctuate significantly include:
| 25 |
| Table of Contents |
|
| ● | the results of operating and financial performance and prospects of other companies in our industry; |
|
|
|
|
|
| ● | strategic actions by us or our competitors, such as acquisitions or restructurings; |
|
|
|
|
|
| ● | the public’s reaction to our press releases, other public announcements, and filings with the Securities and Exchange Commission; |
|
|
|
|
|
| ● | lack of securities analyst coverage or speculation in the press or investment community about us or market opportunities in the smart glass industry; |
|
| ● | changes in government policies in the United States and, as our international business increases, in other foreign countries; |
|
| ● | changes in earnings estimates or recommendations by securities or research analysts who track our common stock or failure of our actual results of operations to meet those expectations; |
|
| ● | market and industry perception of our success, or lack thereof, in pursuing our growth strategy; |
|
| ● | changes in accounting standards, policies, guidance, interpretations or principles; |
|
| ● | any lawsuit involving us, our services or our products; |
|
| ● | arrival and departure of key personnel; |
|
| ● | sales of common stock by us, our investors or members of our management team; |
|
| ● | an announcement that we have effected a reverse split of our common stock; and |
|
| ● | changes in general market, economic and political conditions in the United States and global economies or financial markets, including those resulting from natural or man-made disasters. |
Any of these factors, as well as broader market and industry factors, may result in large and sudden changes in the trading volume of our common stock and could seriously harm the market price of our common stock, regardless of our operating performance. This may prevent you from being able to sell your shares at or above the price you paid for your shares of our common stock, if at all. In addition, following periods of volatility in the market price of a company’s securities, stockholders often institute securities class action litigation against that company. Our involvement in any class action suit or other legal proceeding could divert our senior management’s attention and could adversely affect our business, financial condition, results of operations and prospects.
| 26 |
| Table of Contents |
A large number of shares may be issued and subsequently sold upon the exercise of existing warrants.
As of August 31, 2020, there were 471,605 shares of common stock issuable under outstanding warrants at various exercise prices, this was subsequently reduced to 439,094 shares of common stock issuable under outstanding warrants due to the expiry of 32,511 warrants on October 31, 2020. To the extent that holders of existing warrants sell the shares of common stock issued upon the exercise of warrants, the market price of our common stock may decrease due to the additional selling pressure in the market. The risk of dilution from issuances of shares of common stock underlying existing warrants may cause shareholders to sell their common stock, which could further contribute to any decline in the market price.
We are a “smaller reporting company” under the SEC’s disclosure rules and have elected to comply with the reduced disclosure requirements applicable to smaller reporting companies.
We are a “smaller reporting company” under the SEC’s disclosure rules, meaning that we have either:
|
| · | a public float of less than $250 million; or |
|
|
|
|
|
| · | annual revenues of less than $100 million during the most recently completed fiscal year; and |
|
| · | no public float; or |
|
|
|
|
|
| · | a public float of less than $700 million. |
As a smaller reporting company, we are permitted to comply with scaled-back disclosure obligations in our SEC filings compared to other issuers, including with respect to disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We have elected to adopt the accommodations available to smaller reporting companies. Until we cease to be a smaller reporting company, the scaled-back disclosure in our SEC filings will result in less information about our company being available than for other public companies.
If investors consider our common stock less attractive as a result of our election to use the scaled-back disclosure permitted for smaller reporting companies, there may be a less active trading market for our common stock and our share price may be more volatile.
As a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act.
We are a non-accelerated filer under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and we are not required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. Therefore, our internal controls over financial reporting will not receive the level of review provided by the process relating to the auditor attestation included in annual reports of issuers that are subject to the auditor attestation requirements. In addition, we cannot predict if investors will find our common stock less attractive because we are not required to comply with the auditor attestation requirements. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and trading price for our common stock may be negatively affected.
| 27 |
| Table of Contents |
The speculative nature of our business plan may result in the loss of your investment.
Our operations are in the start-up stage only and are unproven. We may not be successful in implementing our business plan to become profitable. There may be less demand for our services than we anticipate. There is no assurance that our business will succeed and you may lose your entire investment.
Because we do not intend to pay any dividends on our shares, investors seeking dividend income should not purchase our shares.
We have not declared or paid any dividends on our shares since inception, and do not anticipate paying any such dividends for the foreseeable future. We presently do not anticipate that we will pay dividends on any of our common stock in the foreseeable future. If payment of dividends does occur at some point in the future, it would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any dividends on shares of common stock will be within the discretion of our Board. We presently intend to retain all earnings to implement our business plan; accordingly, we do not anticipate the declaration of any dividends for common stock in the foreseeable future.
Investors seeking dividend income should not invest in our shares.
Because we can issue additional shares, purchasers of our shares may incur immediate dilution and may experience further dilution.
We are authorized to issue up to 220,000,000 shares. Our Board has the authority to approve additional share issuances within our authorized share capital without consent of any of our stockholders. Consequently, our stockholders may experience more dilution in their ownership of our Company in the future.
Assuming our securities are listed on Nasdaq, we will incur materially increased costs and become subject to additional regulations and requirements.
As a newly exchange-listed public company, we will incur material additional legal, accounting and other expenses including recruiting and retaining qualified independent directors, payment of annual exchange fees, and satisfying the continued listing standards for Nasdaq. If our common stock and warrants are listed on Nasdaq, we must meet certain financial and liquidity criteria to maintain such listing. If we fail to meet any of Nasdaq’s listing standards, our securities may be delisted. In addition, our Board may determine that the cost of maintaining our listing on a national securities exchange outweighs the benefits of such listing. A delisting of our securities from Nasdaq may materially impair our stockholders’ ability to buy and sell our securities and could have an adverse effect on the market price of, and the efficiency of the trading market for, our securities. The delisting of our securities could significantly impair our ability to raise capital and the value of your investment.
| 28 |
| Table of Contents |
Our by-laws do not contain anti-takeover provisions, which could result in a change of our management and directors if there is a take-over of our company.
We do not currently have a shareholder rights plan or any anti-takeover provisions in our by-laws. Without any anti-takeover provisions, there is no deterrent for a take-over of our Company, which may result in a change in our management and directors.
Our by-laws contain provisions indemnifying our officers and directors against all costs, charges and expenses incurred by them.
Our by-laws contain provisions with respect to the indemnification of our officers and directors against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, actually and reasonably incurred by him, including any amount paid to settle an action or satisfy a judgment in a civil, criminal or administrative action or proceeding to which he is made a party by reason of his being or having been one of our directors or officers.
Risks Related to this Offering and our Reverse Stock Split
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds, including for any of the purposes described in the section of this prospectus entitled “Use of Proceeds.” You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our securities to decline and delay the development of our product candidates. Pending the application of these funds, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
You will experience immediate and substantial dilution in the net tangible book value of the shares you purchase in this offering and may experience additional dilution in the future.
The combined public offering price per share of common stock and related warrant, and the combined public offering price of each pre-funded warrant and related warrant, will be substantially higher than the as adjusted net tangible book value per share of our common stock after giving effect to this offering. Assuming the sale of 1,066,666 shares of our common stock and warrants to purchase up to 1,066,666 shares of common stock at an assumed combined public offering price of $7.50 per share and related warrant, the closing sale price per share of our common stock on the OTCQX on December 31, 2020, on a post reverse split basis, assuming no sale of any pre-funded warrants in this offering, no exercise of the warrants being offered in this offering and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, you will incur immediate dilution of approximately $5.31 per share. As a result of the dilution to investors purchasing securities in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of the liquidation of our company. See the section entitled “Dilution” below for a more detailed discussion of the dilution you will incur if you participate in this offering. To the extent shares are issued under outstanding options and warrants at exercise prices lower than the public offering price of our common stock in this offering, you will incur further dilution.
| 29 |
| Table of Contents |
There is no public market for the pre-funded warrants being offered by us in this offering.
There is no established public trading market for the pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded warrants on any national securities exchange or other nationally recognized trading system. Without an active market, the liquidity of the pre-funded warrants will be limited.
Holders of the warrants offered hereby will have no rights as common stockholders with respect to the shares our common stock underlying the warrants until such holders exercise their warrants and acquire our common stock, except as otherwise provided in the warrants.
Until holders of the warrants and the pre-funded warrants acquire shares of our common stock upon exercise thereof, such holders will have no rights with respect to the shares of our common stock underlying such warrants, except to the extent that holders of such warrants will have certain rights to participate in distributions or dividends paid on our common stock as set forth in the warrants. Upon exercise of the warrants and the pre-funded warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
Even if the reverse stock split achieves the requisite increase in the market price of our common stock for listing of our securities on Nasdaq, we cannot assure you that we will be able to continue to comply with the minimum bid price requirement of Nasdaq.
Even if the reverse stock split achieves the requisite increase in the market price of our common stock to be in compliance with the minimum bid price for listing of our securities on the Nasdaq, there can be no assurance that the market price of our common stock following the reverse stock split will remain at the level required for continuing compliance with that requirement. It is not uncommon for the market price of a company’s common stock to decline in the period following a reverse stock split. If the market price of our common stock declines following the effectuation of the reverse stock split, the percentage decline may be greater than would occur in the absence of a reverse stock split. In any event, other factors unrelated to the number of shares of our common stock outstanding, such as negative financial or operational results, could adversely affect the market price of our common stock and jeopardize our ability to meet or maintain the Nasdaq’s minimum bid price requirement.
| 30 |
| Table of Contents |
Even if the reverse stock split increases the market price of our common stock and we meet the initial listing requirements of Nasdaq, there can be no assurance that we will be able to comply with the continued listing standards of Nasdaq, a failure of which could result in a de-listing of our common stock.
Nasdaq requires that the trading price of its listed stocks remain above one dollar in order for the stock to remain listed. If a listed stock trades below one dollar for more than 30 consecutive trading days, then it is subject to delisting from Nasdaq. In addition, to maintain a listing on Nasdaq, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, and certain corporate governance requirements. If we are unable to satisfy these requirements or standards, we could be subject to delisting, which would have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we would expect to take actions to restore our compliance with the listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the minimum bid price requirement, or prevent future non-compliance with the listing requirements.
The reverse stock split may decrease the liquidity of the shares of our common stock.
The liquidity of the shares of our common stock may be affected adversely by the reverse stock split given the reduced number of shares that will be outstanding following the reverse stock split, especially if the market price of our common stock does not increase as a result of the reverse stock split. In addition, the reverse stock split may increase the number of shareholders who own odd lots (less than 100 shares) of our common stock, creating the potential for such shareholders to experience an increase in the cost of selling their shares and greater difficulty effecting such sales.
Following the reverse stock split, the resulting market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve.
Although we believe that a higher market price of our common stock may help generate greater or broader investor interest, there can be no assurance that the reverse stock split will result in a share price that will attract new investors, including institutional investors. In addition, there can be no assurance that the market price of our common stock will satisfy the investing requirements of those investors. As a result, the trading liquidity of our common stock may not necessarily improve.
| 31 |
| Table of Contents |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. Forward-looking statements involve risks and uncertainties and include statements regarding, among other things, our projected revenue growth and profitability, our growth strategies and opportunity, anticipated trends in our market and our anticipated needs for working capital. They are generally identifiable by use of the words “may,” “will,” “should,” “anticipate,” “estimate,” “plans,” “potential,” “projects,” “continuing,” “ongoing,” “expects,” “management believes,” “we believe,” “we intend” or the negative of these words or other variations on these words or comparable terminology. These statements may be found under the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” as well as in this prospectus generally. In particular, these include statements relating to future actions, prospective products, market acceptance, future performance or results of current and anticipated products, sales efforts, expenses, and the outcome of contingencies such as legal proceedings and financial results.
Examples of forward-looking statements in this prospectus include, but are not limited to, our expectations regarding our business strategy, business prospects, operating results, operating expenses, working capital, liquidity and capital expenditure requirements. Important assumptions relating to the forward-looking statements include, among others, assumptions regarding demand for our products, the cost, terms and availability of components, pricing levels, the timing and cost of capital expenditures, competitive conditions and general economic conditions. These statements are based on our management’s expectations, beliefs and assumptions concerning future events affecting us, which in turn are based on currently available information. These assumptions could prove inaccurate. Although we believe that the estimates and projections reflected in the forward-looking statements are reasonable, our expectations may prove to be incorrect.
Important factors that could cause actual results to differ materially from the results and events anticipated or implied by such forward-looking statements include, but are not limited to:
|
| · | changes in the market acceptance of our products; |
|
|
|
|
|
| · | increased levels of competition; |
|
|
|
|
|
| · | changes in political, economic or regulatory conditions generally and in the markets in which we operate; |
|
|
|
|
|
| · | our relationships with our key customers; |
|
|
|
|
|
| · | our ability to retain and attract senior management and other key employees; |
|
|
|
|
|
| · | our ability to quickly and effectively respond to new technological developments; |
|
|
|
|
|
| · | our ability to protect our trade secrets or other proprietary rights, operate without infringing upon the proprietary rights of others and prevent others from infringing on the proprietary rights of the Company; and |
|
|
|
|
|
| · | other risks, including those described in the “Risk Factors” discussion of this prospectus |
We operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for us to predict all of those risks, nor can we assess the impact of all of those risks on our business or the extent to which any factor may cause actual results to differ materially from those contained in any forward-looking statement. The forward-looking statements in this prospectus are based on assumptions management believes are reasonable. However, due to the uncertainties associated with forward-looking statements, you should not place undue reliance on any forward-looking statements. Further, forward-looking statements speak only as of the date they are made, and unless required by law, we expressly disclaim any obligation or undertaking to publicly update any of them in light of new information, future events, or otherwise.
| 32 |
| Table of Contents |
We estimate that the net proceeds from this offering will be approximately $6,863,093 ($7,995,093 if the underwriters exercise their option to purchase additional securities in full), assuming a combined public offering price per share of common stock and related warrant of $7.50, the closing sale price per share of our common stock on the OTCQX on December 31, 2020, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, and assuming no sale of any fixed combinations of pre-funded warrants and warrants offered hereunder. If the warrants are exercised in full for cash, the estimated net proceeds will increase to $18,863,085(or $21,795,074 if the underwriters’ option to purchase additional securities is exercised in full).
We currently expect to use approximately $2,560,000 of the net proceeds of this offering ($3,700,000 if the underwriters exercise their option to purchase additional securities in full) for research and development studies and the patent and legal costs associated thereto, with the remaining net proceeds to be used for general working capital purposes. If certain investors in our May 2020 private placement participate in this offering, we will pay 8% of the gross proceeds received from these investors to the placement agent for the May 2020 private placement offering.
MARKET FOR OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Market Price for our Common Stock
Our common stock was quoted on the OTCBB and its predecessors under the symbol “LXRA” and then, subsequent to June 2009, under the symbol “LXRP”. On January 4, 2018, the Company’s shares of common stock commenced quotation on the OTCQX. Quotations on the OTCQX reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions. Since October 28, 2009, our common stock has been traded on the Canadian Securities Exchange (CSE) and its predecessors, under the symbol “LXX.”
Holders
As of January 4, 2021 there were approximately 53 stockholders of record holding 3,001,477 shares of our common stock. This number does not include an indeterminate number of stockholders whose shares are held by brokers in street name. The holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. Holders of our common stock have no preemptive rights and no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to our common stock.
Dividend Policy
We have never paid any cash dividends on our common stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements of our business. Any future determination to pay cash dividends will be at the discretion of our Board and will be dependent upon our financial condition, results of operations, capital requirements and such other factors as our Board deems relevant. Our ability to pay cash dividends is subject to limitations imposed by state law.
| 33 |
| Table of Contents |
The following table sets forth our capitalization as of August 31, 2020:
|
| • | on an actual basis; and |
|
| • | on an as adjusted basis to reflect the anticipated reverse stock split of the outstanding common stock of the Company at an assumed 1-for-30 ratio, and the issuance and sale by us of 1,066,666 shares of common stock and warrants to purchase up to 1,066,666 shares of common stock at an assumed combined public offering price of $7.50 per share and related warrant, the closing sale price per share of our common stock on the OTCQX on December 31, 2020, on a post reverse stock split basis, assuming no sale of any pre-funded warrants in this offering, no exercise of the warrants being offered in this offering and accounted for as equity and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
|
|
|
|
|
| • | on a pro forma as adjusted basis giving effect to CanPharm Asset Sale. |
You should consider this table in conjunction with “Use of Proceeds” above as well as our “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the notes to those financial statements for year ended August 31, 2020 included elsewhere in this prospectus.
|
|
| As of August 31, 2020 |
| |||||||||
|
|
| Audited, Actual |
|
| Unaudited, Pro Forma (1) |
|
| Unaudited, Pro Forma As Adjusted (1)(2) |
| |||
| Cash and cash equivalents |
| $ | 1,293,749 |
|
| $ | 1,562,129 |
|
| $ | 8,425,222 |
|
| Total Current Liabilities |
|
| 225,917 |
|
|
| 248,286 |
|
|
| 248,286 |
|
| Total Long-Term Liabilities |
|
| 120,063 |
|
|
| 120,063 |
|
|
| 120,063 |
|
| Stockholders’ Equity (Deficit): |
|
|
|
|
|
|
|
|
|
|
|
|
| Common Stock, $0.001 par value; 220,000,000 authorized; 90,044,312 issued and outstanding pre-split shares as of August 31, 2020, and 3,001,477 issued and outstanding post-split shares as adjusted |
|
| 90,044 |
|
|
| 90,044 |
|
|
| 4,068 |
|
| Additional paid-in capital |
|
| 30,237,355 |
|
|
| 30,237,355 |
|
|
| 37,186,424 |
|
| Accumulated deficit |
|
| (27,802,198 | ) |
|
| (26,405,737 | ) |
|
| (26,405,737 | ) |
| Non-controlling Interest |
|
| (42,943 | ) |
|
| (42,943 | ) |
|
| (42,943 | ) |
| Total Stockholders’ Equity |
| $ | 2,482,258 |
|
| $ | 3,878,719 |
|
| $ | 10,741,812 |
|
| 34 |
| Table of Contents |
(1) Refers to the CanPharm Asset Sale, which occurred on November 18, 2020 and closed on December 9, 2020 . As of the closing on December 9, 2020 , the Company and CanPharm have no part or interest in those ongoing revenue streams from the license agreements. Pursuant to the CanPharm Asset Sale, CanPharm received CDN$350,000 in cash at closing . Additionally, Hill Street has agreed to issue to CanPharm CDN$1.5 million of Hill Street Shares over 3 milestone tranches, of which 6,031,363 Hill Street Shares (equal in value to CDN$500,000) were issued to CanPharm at closing, as well as a CDN$2 million promissory note included at its nominal value ($NIL) that bears interest at 10% per annum and is repayable in quarterly installments based on 5% of Hill Street’s gross revenues derived from products utilizing the intellectual property until fully paid.
(2) A 50% increase or decrease in the assumed combined public offering price per share of common stock and related warrant would increase or decrease each of cash, common stock and additional paid-in capital, total stockholders’ equity and total capitalization on an as adjusted basis by approximately $3.76 million, assuming that the number of shares, pre-funded warrants and warrants offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. An increase or decrease of 50% in the number of shares of common stock (or common stock underlying pre-funded warrants) and related warrants offered by us, as set forth on the cover page of this prospectus, would increase or decrease each of cash, common stock and additional paid-in capital, total stockholders’ equity and total capitalization on an as adjusted basis by approximately $ 3.6 million, assuming no change in the assumed combined public offering price per share of common stock and related warrant after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The above discussion and table are based on 90,044,312 pre-split shares outstanding as of August 31, 2020, 3,001,477 as adjusted, and actual numbers do not give effect to our planned reverse stock split. The discussion and table do not include, as of that date:
|
| • | 14,148,154 pre-split shares, 471,605 shares as adjusted, of our common stock issuable upon exercise of outstanding warrants, at a weighted average exercise price of $0.56 per share or 16.77 per share as adjusted; |
|
| • | 5,148,000 pre-split shares, 171,600 shares as adjusted, of our common stock issuable upon exercise of outstanding options at a weighted average exercise price of $0.37 per share or $11.17 per share as adjusted; and |
|
| • | shares of common stock that may be issued upon exercise of the underwriters’ warrants issued in this offering. |
| 35 |
| Table of Contents |
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the assumed combined public offering price per share of common stock and related warrant and as adjusted, net tangible book value per share of common stock immediately after this offering.
Our net tangible book value is the amount of our total tangible assets less our total liabilities. Our net tangible book value as of August 31, 2020 was $2,063,338, or $0.69 per share of common stock. After giving effect to the assumed sale of 1,066,666 shares of our common stock and warrants to purchase up to 1,066,666 shares of common stock at an assumed combined public offering price of $7.50 per share and related warrant, the closing sale price per share of our common stock on the OTCQX on December 31, 2020, assuming no sale of any pre-funded warrants in this offering, no exercise of the warrants being offered in this offering and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted, net tangible book value per share as of August 31, 2020, would have been approximately $8,926,093, or approximately $2.19 per share. This represents an immediate increase in net tangible book value per share of $1.50 to existing stockholders and an immediate dilution of approximately $5.31 per share to new investors purchasing shares of our common stock in this offering.
Dilution per share to new investors is determined by subtracting the as adjusted, net tangible book value per share after this offering from the combined public offering price per share and related warrant paid by new investors.
The following table illustrates this per share dilution:
| Assumed combined public offering price per share and related warrant |
| $ | 7.50 |
|
| Net tangible book value per share as of August 31, 2020 |
| $ | 0.69 |
|
| Increase in as adjusted net tangible book value per share after this offering |
| $ | 1.50 |
|
| As adjusted, net tangible book value per share after giving effect to this offering |
| $ | 2.19 |
|
| Dilution per share to new investors |
| $ | 5.31 |
|
A 50% increase (decrease) in the assumed combined public offering price per share of common stock and related warrant would increase (decrease) the as adjusted, net tangible book value per share by $1.11 ($0.89), and the dilution per share to new investors in this offering by $4.20($6.20), assuming the number of common stock, pre-funded warrants and warrants offered by us, as set forth on the cover page of this prospectus, remain the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
Conversely, a decrease of 50% in the number of shares of common stock (or common stock underlying pre-funded warrants) and related warrants offered by us, as set forth on the cover page of this prospectus, would decrease the as adjusted, net tangible book value by approximately $0.70 per share and increase the dilution to investors participating in this offering by approximately $0.70 per share, assuming the assumed combined public offering price per share of common stock and related warrant remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The information above assumes that the underwriters do not exercise their option to purchase additional securities. If the underwriters exercise their option to purchase additional securities in full, the as adjusted, net tangible book value will increase to $2.80 per share, representing an immediate increase to existing stockholders of $2.11 per share and an immediate dilution of $5.39 per share to new investors.
| 36 |
| Table of Contents |
The foregoing discussion and table do not take into account further dilution to new investors that could occur upon the exercise of outstanding warrants having a per share exercise or conversion price less than the per share offering price to the public in this offering.
We may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
The above discussion and table are based on 90,044,312 pre-split shares outstanding as of August 31, 2020, without giving effect to our planned reverse stock split, and 3,001,477 as adjusted for the reverse stock split at an assumed 1-for-30 ratio. The discussion and table do not include, as of that date:
|
| •
| 14,148,154 pre-split shares, 471,605 shares as adjusted for the reverse stock split at an assumed 1-for-30 ratio of our common stock issuable upon exercise of outstanding warrants, at a weighted average exercise price of $0.56 per share or $16.77 per share as adjusted for the reverse stock split at an assumed 1-for-30 ratio; and |
|
| • | 5,148,000 pre-split shares, 171,600 shares as adjusted, of our common stock issuable upon exercise of outstanding options at a weighted average exercise price of $0.37 per share or $ 11.17 per share as adjusted for the reverse stock split at an assumed 1-for-30 ratio. |
| 37 |
| Table of Contents |
UNAUDITED PRO FORMA COMBINED FINANCIAL INFORMATION
The following unaudited pro forma combined financial statements were prepared by applying certain pro forma adjustments to the historical financial statements of the Company. The pro forma adjustments give effect to the CanPharm Asset Sale.
The unaudited pro forma combined statements of operations for our fiscal years ended August 31, 2020 and 2019, respectively, give effect to the CanPharm Asset Sale as if it had occurred on September 1, of each period.
These pro forma combined financial statements include adjustments for the CanPharm Asset Sale which closed on December 9, 2020, assuming our public offering price is $7.50 per share and related warrant.
We determined that the CanPharm Asset Sale, considering the guidance in Rule 11-01 (b) of Regulation S-X, met the significance test of Rule 3-05 of Regulation S-X.
These unaudited pro forma combined financial statements do not purport to represent what our results of operations or financial condition would have been had the CanPharm Asset Sale actually occurred on the assumed dates, nor do they purport to project our results of operations or financial condition for any future period or future date. You should read these unaudited pro forma combined financial statements in conjunction with “Capitalization,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the historical financial statements, including the related notes thereto, appearing elsewhere in this prospectus.
The following table includes (i) summary consolidated statement of operations data for the years ended August 31, 2020 and 2019 and (ii) summary consolidated balance sheet data as of August 31, 2020 and August 31, 2019 (unaudited), derived from our audited consolidated financial statements and related notes included elsewhere in this prospectus. Our financial statements are prepared and presented in accordance with generally accepted accounting principles in the United States. The results indicated below are not necessarily indicative of our future performance.
You should read this information together with the sections entitled “Capitalization”, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this prospectus.
| UNAUDITED PRO FORMA CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS | ||||||||||||||||||||||||||||||||
| (Expressed in U.S. Dollars, except number of shares) | ||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||
|
|
| Reported |
|
|
|
|
|
| Pro Forma |
|
| Reported |
|
|
|
|
|
| Pro Forma |
| ||||||||||||
|
|
| August 31 |
|
| Pro Forma |
|
|
|
| August 31 |
|
| August 31 |
|
| Pro Forma |
|
|
|
| August 31 |
| ||||||||||
|
|
| 2020 |
|
| Adjustments |
|
| Notes |
|
| 2020 |
|
| 2019 |
|
| Adjustments |
|
| Notes |
|
| 2019 |
| ||||||||
|
|
| (Audited) |
|
|
|
|
|
| (Unaudited) |
|
| (Audited) |
|
|
|
|
|
| (Unaudited) |
| ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
| Revenue |
| $ | 384,543 |
|
| $ | (156,750 | ) |
|
| (1) |
| $ | 227,793 |
|
| $ | 222,610 |
|
| $ | (91,000 | ) |
|
| (1) |
| $ | 131,610 |
| ||
| Cost of Goods Sold |
|
| 99,378 |
|
|
|
|
|
|
|
|
|
|
| 99,378 |
|
|
| 22,893 |
|
|
|
|
|
|
|
|
|
|
| 22,893 |
|
| Gross profit |
|
| 285,165 |
|
|
|
|
|
|
|
|
|
|
| 128,415 |
|
|
| 199,717 |
|
|
|
|
|
|
|
|
|
|
| 108,717 |
|
| Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accounting and audit |
|
| 78,650 |
|
|
|
|
|
|
|
|
|
|
| 78,650 |
|
|
| 77,388 |
|
|
|
|
|
|
|
|
|
|
| 77,388 |
|
| Depreciation and amortization |
|
| 112,750 |
|
|
|
|
|
|
|
|
|
|
| 112,750 |
|
|
| 60,550 |
|
|
|
|
|
|
|
|
|
|
| 60,550 |
|
| Advertising and promotions |
|
| 204,277 |
|
|
| (45,605 | ) |
|
| (1) |
|
| 158,672 |
|
|
| 515,360 |
|
|
| (1,098 | ) |
|
| (1) |
|
| 514,262 |
| ||
| Bad debt |
|
| 50,000 |
|
|
| (25,000 | ) |
|
| (1) |
|
| 25,000 |
|
|
| 75,000 |
|
|
|
|
|
|
|
|
|
|
| 75,000 |
| |
| Consulting |
|
| 2,193,076 |
|
|
|
|
|
|
|
|
|
|
| 2,193,076 |
|
|
| 1,444,735 |
|
|
|
|
|
|
|
|
|
|
| 1,444,735 |
|
| Investor relation |
|
| 184,277 |
|
|
|
|
|
|
|
|
|
|
| 184,277 |
|
|
| 203,893 |
|
|
|
|
|
|
|
|
|
|
| 203,893 |
|
| Legal and professional |
|
| 371,844 |
|
|
|
|
|
|
|
|
|
|
| 371,844 |
|
|
| 670,863 |
|
|
|
|
|
|
|
|
|
|
| 670,863 |
|
| Office and miscellaneous |
|
| 292,880 |
|
|
| 12,978 |
|
|
| (1) |
|
| 305,858 |
|
|
| 297,209 |
|
|
| (14,226 | ) |
|
| (1) |
|
| 282,983 |
| ||
| Research and development |
|
| 387,074 |
|
|
|
|
|
|
|
|
|
|
| 387,074 |
|
|
| 555,730 |
|
|
|
|
|
|
|
|
|
|
| 555,730 |
|
| Travel |
|
| 47,336 |
|
|
| (672 | ) |
|
| (1) |
|
| 46,664 |
|
|
| 100,587 |
|
|
|
|
|
|
|
|
|
|
| 100,587 |
| |
| Wages and salaries |
|
| 401,283 |
|
|
|
|
|
|
|
|
|
|
| 401,283 |
|
|
| 333,199 |
|
|
|
|
|
|
|
|
|
|
| 333,199 |
|
| Loss on disposal of marketable securities |
|
| 18,198 |
|
|
|
|
|
|
|
|
|
|
| 18,198 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
|
| Unrealized (gain)/loss on marketable securities |
|
| 19,893 |
|
|
|
|
|
|
|
|
|
|
| 19,893 |
|
|
| 16,434 |
|
|
|
|
|
|
|
|
|
|
| 16,434 |
|
| Inventory writeoff |
|
| 8,240 |
|
|
|
|
|
|
|
|
|
|
| 8,240 |
|
|
| 7,182 |
|
|
|
|
|
|
|
|
|
|
| 7,182 |
|
|
|
|
| 4,369,778 |
|
|
|
|
|
|
|
|
|
|
| 4,311,479 |
|
|
| 4,358,130 |
|
|
|
|
|
|
|
|
|
|
| 4,342,806 |
|
| Net loss from continuing operations |
|
| (4,084,613 | ) |
|
|
|
|
|
|
|
|
|
| (4,183,064 | ) |
|
| (4,158,413 | ) |
|
|
|
|
|
|
|
|
|
| (4,234,089 | ) |
| Discontinued operations |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income from discontinued operations |
|
| - |
|
|
| 98,451 |
|
|
| (1) |
|
| 98,451 |
|
|
| - |
|
|
| 75,676 |
|
|
| (1) |
|
| 75,676 |
| ||
| Gain/(Loss) on disposal |
|
| - |
|
|
| 1,396,461 |
|
|
| (1) |
|
| 1,396,461 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
| |
| Gain/(Loss) on discontinued operations |
|
| - |
|
|
|
|
|
|
|
|
|
|
| 1,494,912 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| 75,676 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss and comprehensive loss for the period |
| $ | (4,084,613 | ) |
|
|
|
|
|
|
|
|
| $ | (2,688,152 | ) |
| $ | (4,158,413 | ) |
|
|
|
|
|
|
|
|
| $ | (4,158,413 | ) |
| Net loss and comprehensive loss attributable to: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common shareholders |
| $ | (3,933,996 | ) |
|
|
|
|
|
|
|
|
| $ | (2,537,535 | ) |
| $ | (4,099,420 | ) |
|
|
|
|
|
|
|
|
| $ | (4,099,420 | ) |
| Non-controlling interest |
| $ | (150,617 | ) |
|
|
|
|
|
|
|
|
| $ | (150,617 | ) |
| $ | (58,993 | ) |
|
|
|
|
|
|
|
|
| $ | (58,993 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Basic and diluted loss per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Continuing operations |
| $ | (0.05 | ) |
|
|
|
|
|
|
|
|
| $ | (1.51 | ) |
| $ | (0.05 | ) |
|
|
|
|
|
|
|
|
| $ | (1.63 | ) |
| Discontinued |
| $ | - |
|
|
|
|
|
|
|
|
|
| $ | 0.54 |
|
| $ | - |
|
|
|
|
|
|
|
|
|
| $ | 0.03 |
|
| Total |
| $ | (0.05 | ) |
|
|
|
|
|
|
|
|
| $ | (0.97 | ) |
| $ | (0.05 | ) |
|
|
|
|
|
|
|
|
| $ | (1.60 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average number of common shares outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| - Basic and diluted |
|
| 83,201,271 |
|
|
|
|
|
|
|
|
|
|
| 2,773,376 |
|
|
| 77,792,263 |
|
|
|
|
|
|
|
|
|
|
| 2,593,076 |
|
| 38 |
| Table of Contents |
| UNAUDITED PRO FORMA CONSOLIDATED BALANCE SHEET | ||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||
|
|
| As Reported |
|
|
|
|
|
|
|
| Pro Forma |
|
| As Reported |
|
|
|
|
|
|
|
| Pro Forma |
| ||||||||
|
|
| August 31 |
|
| Pro Forma |
|
|
|
|
| August 31 |
|
| August 31 |
|
| Pro Forma |
|
|
|
|
| August 31 |
| ||||||||
|
|
| 2020 |
|
| Adjustments |
|
| Notes |
|
| 2020 |
|
| 2019 |
|
| Adjustments |
|
| Notes |
|
| 2019 |
| ||||||||
| Current Assets |
| (Audited) |
|
|
|
|
|
|
|
| (Unaudited) |
|
| (Audited) |
|
|
|
|
|
|
|
| (Unaudited) |
| ||||||||
| Cash and cash equivalents |
| $ | 1,293,749 |
|
| $ | 7,131,473 |
|
|
| (2) |
| $ | 8,425,222 |
|
| $ | 1,285,147 |
|
|
|
|
|
|
|
| $ | 1,285,147 |
| |||
| Marketable securities |
|
| 19,321 |
|
|
| 383,400 |
|
|
| (1) |
|
| 402,721 |
|
|
| 64,214 |
|
|
|
|
|
|
|
|
| 64,214 |
| |||
| Accounts receivable |
|
| 313,925 |
|
|
| 278,400 |
|
|
| (1) |
|
| 592,325 |
|
|
| 273,145 |
|
|
| (81,000 | ) |
|
| (1) |
|
| 192,145 |
| ||
| Inventory |
|
| 116,871 |
|
|
|
|
|
|
|
|
|
|
| 116,871 |
|
|
| 127,396 |
|
|
|
|
|
|
|
|
|
|
| 127,396 |
|
| Prepaid expenses and deposit |
|
| 182,095 |
|
|
|
|
|
|
|
|
|
|
| 182,095 |
|
|
| 68,927 |
|
|
|
|
|
|
|
|
|
|
| 68,927 |
|
| Current assets from discontinued operations |
|
|
|
|
|
| 105,250 |
|
|
| (1) |
|
| 105,250 |
|
|
|
|
|
|
| 81,000 |
|
|
| (1) |
|
| 81,000 |
| ||
| Total Current Assets |
|
| 1,925,961 |
|
|
|
|
|
|
|
|
|
|
| 9,824,484 |
|
|
| 1,818,829 |
|
|
|
|
|
|
|
|
|
|
| 1,818,829 |
|
| Non-current assets, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Intellectual property |
|
| 292,000 |
|
|
|
|
|
|
|
|
|
|
| 292,000 |
|
|
| 265,127 |
|
|
|
|
|
|
|
|
|
|
| 265,127 |
|
| Lease right of use |
|
| 126,920 |
|
|
|
|
|
|
|
|
|
|
| 126,920 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
|
| Long Term Receivable |
|
| - |
|
|
| 383,400 |
|
|
| (1) |
|
| 383,400 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
| |
| Property & equipment |
|
| 483,357 |
|
|
|
|
|
|
|
|
|
|
| 483,357 |
|
|
| 591,263 |
|
|
|
|
|
|
|
|
|
|
| 591,263 |
|
| Total Non-Current Assets |
|
| 902,277 |
|
|
|
|
|
|
|
|
|
|
| 1,285,677 |
|
|
| 856,390 |
|
|
|
|
|
|
|
|
|
|
| 856,390 |
|
| TOTAL ASSETS |
| $ | 2,828,238 |
|
|
|
|
|
|
|
|
|
| $ | 11,110 ,161 |
|
| $ | 2,675,219 |
|
|
|
|
|
|
|
|
|
| $ | 2,675,219 |
|
| Current Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
| $ | 86,920 |
|
| $ | 22,119 |
|
|
| (1) |
| $ | 109,039 |
|
| $ | 136,411 |
|
| $ | (320 | ) |
|
| (1) |
| $ | 136,091 |
| ||
| Deferred revenue |
|
| 44,255 |
|
|
|
|
|
|
|
|
|
|
| 44,255 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
|
| Due to related party |
|
| 58,704 |
|
|
|
|
|
|
|
|
|
|
| 58,704 |
|
|
| 48,096 |
|
|
|
|
|
|
|
|
|
|
| 48,096 |
|
| Lease current |
|
| 36,038 |
|
|
|
|
|
|
|
|
|
|
| 36,038 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
|
| Liabilities from discontinued operations |
|
|
|
|
|
| 250 |
|
|
| (1) |
|
| 250 |
|
|
|
|
|
|
| 320 |
|
|
| (1) |
|
| 320 |
| ||
| Total Current Liabilities |
|
| 225,917 |
|
|
|
|
|
|
|
|
|
|
| 248,286 |
|
|
| 184,507 |
|
|
|
|
|
|
|
|
|
|
| 184,507 |
|
| Long Term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lease long term |
|
| 89,393 |
|
|
|
|
|
|
|
|
|
|
| 89,393 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
|
| Loan payable |
|
| 30,670 |
|
|
|
|
|
|
|
|
|
|
| 30,670 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
|
| Total Long Term Liabilities |
|
| 120,063 |
|
|
|
|
|
|
|
|
|
|
| 120,063 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
| - |
|
| TOTAL LIABILITIES |
|
| 345,980 |
|
|
|
|
|
|
|
|
|
|
| 368,349 |
|
|
| 184,507 |
|
|
|
|
|
|
|
|
|
|
| 184,507 |
|
| STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Share Capital |
|
| 90,044 |
|
|
| (86,055 | ) |
|
| (1)(2) |
|
| 4,068 |
|
|
| 78,787 |
|
|
| (76,161 | ) |
|
| (2) |
|
| 2,626 |
| ||
| Additional paid-in capital |
|
| 30,237,355 |
|
|
| 6,949,148 |
|
|
| (2) |
|
| 37,186,424 |
|
|
| 26,172,453 |
|
|
| 76,161 |
|
|
| (2) |
|
| 26,248,614 |
| ||
| Deficit |
|
| (27,802,198 | ) |
|
| 1,396,461 |
|
|
| (2) |
|
| (26,405,737 | ) |
|
| (23,868,202 | ) |
|
|
|
|
|
|
|
|
|
| (23,868,202 | ) | |
| Equity attributable to shareholders of the Company |
|
| 2,525,201 |
|
|
|
|
|
|
|
|
|
|
| 10,784,75 5 |
|
|
| 2,383,038 |
|
|
|
|
|
|
|
|
|
|
| 2,383,038 |
|
| Non-Controlling Interest |
|
| (42,943 | ) |
|
|
|
|
|
|
|
|
|
| (42,943 | ) |
|
| 107,674 |
|
|
|
|
|
|
|
|
|
|
| 107,674 |
|
| Total Stockholders' Equity |
|
| 2,482,258 |
|
|
|
|
|
|
|
|
|
|
| 10,741,812 |
|
|
| 2,490,712 |
|
|
|
|
|
|
|
|
|
|
| 2,490,712 |
|
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
| $ | 2,828,238 |
|
|
|
|
|
|
|
|
|
| $ | 11,110,161 |
|
| $ | 2,675,219 |
|
|
|
|
|
|
|
|
|
| $ | 2,675,219 |
|
Pro Forma Adjustments:
(1) Refers to the CanPharm Asset Sale, which occurred on November 18, 2020 and closed on December 9, 2020 . As of the closing on December 9, 2020 , the Company and CanPharm have no part or interest in those ongoing revenue streams from the license agreements. Pursuant to the CanPharm Asset Sale, CanPharm received CDN$350,000 in cash at closing . Additionally, Hill Street has agreed to issue to CanPharm CDN$1.5 million of Hill Street shares over 3 milestone tranches, of which 6,031,363 Hill Street Shares (valued at CDN$500,000) were issued to CanPharm at closing, and a CDN$2 million promissory note at closing included at its nominal value ($NIL) that bears interest at 10% per annum and is repayable in quarterly installments based on 5% of Hill Street’s gross revenues derived from products utilizing the intellectual property until fully paid.
(2) A 50% increase or decrease in the assumed combined public offering price per share of common stock and related warrant would increase or decrease each of cash, common stock and additional paid-in capital, total stockholders’ equity and total capitalization on an as adjusted basis by approximately $3,640,000, assuming that the number of shares, pre-funded warrants and warrants offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discount and estimated offering expenses payable by us. An increase or decrease of 50% in the number of shares of common stock (or common stock underlying pre-funded warrants) and related warrants offered by us, as set forth on the cover page of this prospectus, would increase or decrease each of cash, common stock and additional paid-in capital, total stockholders’ equity and total capitalization on an as adjusted basis by approximately $3,760,000, assuming no change in the assumed combined public offering price per share of common stock and related warrant after deducting the underwriting discount and estimated offering expenses payable by us.
| 39 |
| Table of Contents |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
Our financial statements are prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). These accounting principles require us to make certain estimates, judgments and assumptions. We believe that the estimates, judgments and assumptions upon which we rely are reasonable based upon information available to us at the time that these estimates, judgments and assumptions are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities as of the date of the financial statements as well as the reported amounts of revenues and expenses during the periods presented. Our financial statements would be affected to the extent there are material differences between these estimates and actual results. In many cases, the accounting treatment of a particular transaction is specifically dictated by GAAP and does not require management’s judgment in its application. There are also areas in which management’s judgment in selecting any available alternative would not produce a materially different result. The following discussion should be read in conjunction with our financial statements and notes thereto appearing elsewhere in this Registration Statement on Form S-1.
General and Historical Overview of Our Business
The Company was formed on December 9, 2004 under the laws of the State of Nevada. In March of 2014, the Company began work in the fields of enhanced delivery of active ingredients and drugs. The Company filed its first patent application in 2014 and today has over 50 patent applications pending around the world, with 18 patents granted to date, all related to its DehydraTECHTM technology (“DehydraTECH”) and certain characteristics of oral ingredient and drug delivery. Additional early stage investigation has been conducted of topically-administered products such as patches, creams and lotions.
The Company’s patent applications developed from its Research and Development programs (“R&D”) currently include, but are not limited to, fat-soluble versions of vitamins, NSAIDs, nicotine, hormones, phosphodiesterase inhibitors, and antivirals. 2018 animal studies demonstrated a propensity for its DehydraTECH technology to elevate the quantity of nicotine delivered across the blood-brain-barrier. This expanded our patent applications and opened possibilities for improved delivery of certain central nervous system-targeted drugs that require additional R&D.
In a 12-participant human clinical study performed in 2018 and published in 2019 in a peer reviewed medical journal, Advances in Therapy titled “Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study” available on the PubMed.gov website with the identification of PMID: 31512143, the Company demonstrated that its technology delivered higher volumes of cannabidiol into the human circulatory system and did so more quickly than a concentration-matched positive control. This same study also demonstrated a statistically significant reduction in human blood pressure from the DehydraTECH processed cannabidiol, versus no statistical reduction in human blood pressure from the positive control.
In the Company’s most recent animal study conducting research on DehydraTECH within two classes of antiviral therapies (a Protease Inhibitor and a Reverse Transcriptase Inhibitor) which are currently in use against HIV/AIDS and are being investigated for use against SARS-CoV-2/COVID-19, noted improvements in the delivery of the antiviral drugs in the animal bloodstream were demonstrated. The improved delivery of the antivirals along with the animal’s demonstrated safety and tolerability of the DehydraTECH formulations has led the Company to begin preparations for expanded investigations into antiviral drug delivery enhancement and effectiveness and filing additional patent applications.
We operate a Health Canada-licensed laboratory located in Kelowna, British Columbia to conduct basic research and formulation operations, and typically outsource virtually all analytical work to independent third-party laboratories located in Canada, the USA, and Europe. Such third-party evaluation provides independent assessment of the effects of our technology and processes.
| 40 |
| Table of Contents |
The Company’s formulation and process-oriented operations are primarily conducted in its own laboratory and validated through third-party testing, in preparation for partnering with industry leaders for adoption into their consumer products and/or drugs. Other than for R&D purposes and the limited production of CBD infused products which are currently in the process of being wound down, the Company does not produce, manufacture, market, sell or distribute drugs.
Our common stock is quoted on the OTCQX under the symbol "LXRP" and on the Canadian Securities Exchange under the symbol “LXX”.
We maintain our registered agent's office and our U.S. business office at Nevada Agency and Transfer Company, 50 West Liberty, Suite 880, Reno, Nevada 89501. Our telephone number is (755) 322-0626.
The address of our principal executive office is Unit 100–740 McCurdy Road, Kelowna BC V1X 2P7. We have administrative functions located in Phoenix, Arizona. Our main corporate website is located at www.lexariabioscience.com.
Due to the implementation of British Columbia Instrument 51-509 on September 30, 2008, by the British Columbia Securities Commission (“BCSC”), we have been deemed to be a British Columbia based reporting issuer. As such, we are required to file certain information and documents at www.sedar.com.
Plan of Operation
During the next twelve-month period (beginning September 1, 2020), we intend to:
|
| · | pursue a listing on a U.S. senior stock exchange or market; |
|
|
|
|
|
| · | pursue technology out-licensing opportunities for our patented DehydraTECH technology. This will be focused first primarily on the CBD-from-hemp and the nicotine sectors, respectively, and will evolve as time allows for completed R&D in the NSAID sector, and will eventually include the anti viral drug sectors if and as our R&D supports such initiatives; |
|
|
|
|
|
| · | identify and secure sources of equity and/or debt financing for intellectual property pursuit and maintenance, R&D, and consumer product formulation and marketing and general corporate operations; |
Our plans beyond fiscal 2021 are dependent upon our ability to obtain sufficient capital through equity capital or other finance choices and by revenues generation which we expect to improve slightly. During the previous year we did raise sufficient capital to fulfill all our plans. Without sufficient capital, our plans will change, and could change materially. We anticipate that we will incur up to the following operating expenses during this period:
| Estimated Funding Required During the 12 Months beginning September 1, 2020 | |||||||
| Expense |
| Amount ($) |
|
| Estimated Completion/Due Date | ||
| Research and Development (Products) Research and Development (General) |
| 50,000 600,000 |
|
| 12 months 12 months | ||
| Patent applications and trademark |
|
| 300,000 |
|
| 12 months | |
| Marketing and Sales |
|
| 75,000 |
|
| 12 months | |
| Consulting Fees (~50% in officers and directors contracts) |
|
| 1,200,000 |
|
| 12 months | |
| Wages and Salaries |
|
| 525,000 |
|
| 12 months | |
| Professional Fees |
|
| 200,000 |
|
| 12 months | |
| Rent |
|
| 45,000 |
|
| 12 months | |
| Other general administrative expenses (including travel, insurance, conferences, and fees) |
|
| 300,000 |
|
| 12 months | |
| Interest Expense |
|
| 10,000 |
|
| 12 months | |
| Total |
|
| 3,305,000 |
|
|
| |
| 41 |
| Table of Contents |
12 Month Outlook for Current Product Line, Product Development & Design, Patents
As at August 31, 2020, we had a working capital surplus of $1,700,044 and cash on hand of $1,293,749. We therefore estimate that we will require approximately $2.0 million in cash to finance our planned expenditures for the 12 months beginning September 1, 2020. In the uncertain event that we are unable to raise sufficient funds to execute our current business plan, we will scale back our operations to prioritize immediate and necessary expenses, shifting portions of our plan into our longer term planning for fiscal 2022. We estimate our minimum necessary expenses for the year to be roughly $2.5 million in which case we would require approximately $1.2 million in additional financed cash to meet our minimum level of expenditures. These necessary expenses include professional fees, wages and general and administrative expenses necessary to satisfy our public reporting requirements.
Our business strategy involves several elements and has evolved from recent years. We intend to prioritize our revenue generating efforts in 2021/22 on our Technology licensing in the nicotine and pharmaceutical sectors, respectively, with a secondary focus on expanding our R&D to support applications of DehydraTECH for drug and related active ingredient delivery.
We developed DehydraTECH to aid absorption and bioavailability of certain “payload” molecules, including, but not limited to, nicotine, NSAIDs and lipophilic vitamins – all of which have received granted patents. DehydraTECH appears to improve absorption and bioavailability of cannabinoids and nicotine into human epi-intestinal cells. In order to market the efficacy of DehydraTECH to potential licensees for use in their product development and manufacturing, we developed a line of oral delivered consumer products. Our consumer products helped us establish modest brand recognition for the Company and DehydraTECH and greatly improved our knowledge of how to implement DehydraTECH into consumer products. In addition, developing such consumer products has aided in our understanding of some CPG manufacturing processes and has helped us understand the needs of potential corporate licensees of DehydraTECH.
The development of consumer products was a minor part of our historical business practice and does not form a part of our current business practice. Any historical product lines that have been developed by the Company are intended to be phased out within the next six months.
The main focus of our current business strategy is the development and out-licensing of DehydraTECH. The out-licensing of DehydraTECH comprised the largest portion of our revenue in our fiscal year ended August 31, 2020.
Pursuant to the disposition of CanPharm’s assets to Hill Street Beverage Company Inc., namely the ability to use and sub-license the use of DehydraTECH with non-pharmaceutical consumer products containing greater than 0.3% THC, we no longer directly license DehydraTECH for use in THC-containing products. However, we do maintain our right, via our subsidiary Lexaria Pharmaceutical Corp., to use and sub-license the use of DehydraTECH with pharmaceutical products that are intended to be designated as a drug which contain greater than 0.3% THC. Our primary business focus is no longer related to cannabinoids, even though that sector is where our Technology was originally developed.
We have retained a limited license to use our Technology to produce THC products operating in international jurisdictions where doing so is legal, however, we currently do not have any plans to produce such products.
We are now focused on incorporating our Technology with other molecules such as nicotine and have licensed our Technology to Altria Ventures Inc., an indirect wholly-owned subsidiary of Altria Group, Inc. Our October 31, 2017 announcement of the USPTO Notice of Allowance for our first patent granted and the subsequent 17 granted patents of our Technology related to new molecule groups, along with our ongoing patent filing and grants, may enhance our ability to successfully pursue this initiative during fiscal 2021 and beyond.
| 42 |
| Table of Contents |
We expect to devote an unknown but increasing proportion of our resources and focus towards pharmaceutical applications and launched operations in this division during our 2021 fiscal year. Our past R&D in other sectors has contributed greatly to our understanding of DehydraTECH and has encouraged us to attempt to reach more meaningful commercial applications in the pharmaceutical sector than were available in cannabinoid sector.
We continue to communicate the potential benefits of DehydraTECH to potential licensing partners, i.e. with higher absorption levels, a manufacturer could perhaps infuse smaller amounts of active molecules into a product, thus reducing their manufacturing input costs, to provide higher bioavailability with the dosing limits being imposed or contemplated in many jurisdictions, to infuse consumer products while masking the flavor and smell of the active molecules, and predictable delivery times. We believe these to be meaningful competitive advantages that may lead to the potential to generate licensing revenue, and will pursue these opportunities within the cannabinoids, nicotine and other bioactive molecular markets both within the USA and internationally in those jurisdictions where they are legal and regulated by government.
We do not sell any nicotine products and do not intend to – however our joint venture partner or other companies active in the tobacco or nicotine sectors may elect to utilize our Technology in products containing non-combustible nicotine for sale to consumers in the USA or internationally.
Subject to budgetary availability, we also plan to conduct additional in vitro and in vivo studies testing the absorption of some or all of the molecules named within our patent applications – NSAIDs, Vitamins, CBD, PDE5 inhibitors, Nicotine and anti viral drugs – to substantiate the effectiveness of DehydraTECH. More than satisfying scientific curiosity, successful tests could potentially lead to increased awareness and acceptance of DehydraTECH as a meaningful method by which to deliver some or all of the named molecules more effectively than their current delivery methods. Therefore, absorption tests could become an important element leading towards higher rates of acceptance of our technology licensing initiatives.
We will pursue technology licensing opportunities as a method of generating highly profitable revenue streams over long periods of time. In addition, while nine of our US patent applications, one of our European patent applications, and eight of our Australian patent applications have been granted to date, we have multiple other patent applications filed in the US and around the world. It is not possible to forecast with certainty when, or if, our remaining pending patent applications will become granted patents. But, if our remaining patent applications do become granted patents, our ability to generate meaningful license revenue from our intellectual property may increase in a short period of time.
We will continue to pursue our remaining patents pending as vigorously as we are able, since the successful granting of more of those applications could lead to material increases in shareholder value. We are currently pursuing patent protection in nine countries around the world but can potentially pursue up to 153 countries via our pending Patent Cooperation Treaty (“PCT”) applications.
Results of Operations
Results of Operations for our Year Ended August 31, 2020 and August 31, 2019
Our net loss and comprehensive loss for the year ended August 31, 2020, for the year ended August 31, 2019 and the changes between those periods for the respective items are summarized as follows:
| 43 |
| Table of Contents |
|
|
| YEAR ENDED |
|
|
| |||||||
|
|
| August 31 |
|
| August 31 |
|
|
| ||||
|
|
| 2020 |
|
| 2019 |
|
| Change |
| |||
|
|
| $ |
|
| $ |
|
| $ |
| |||
| Revenue |
|
| 384,543 |
|
|
| 222,610 |
|
|
| 161,933 |
|
| Consulting fees & employees |
|
| 2,594,359 |
|
|
| 1,777,934 |
|
|
| 816,425 |
|
| Legal and professional |
|
| 371,844 |
|
|
| 670,863 |
|
|
| (299,019 | ) |
| Other general and administrative |
|
| 1,403,575 |
|
|
| 1,909,333 |
|
|
| (505,758 | ) |
| Net Loss |
|
| (4,084,613 | ) |
|
| (4,158,413 | ) |
|
| 73,800 |
|
Revenue
Licensing revenues represent the majority of the $384,543 in revenues during the year ended August 31, 2020 and include a significant increase in product revenues from the sale of our intermediary products. Licensing revenue increases were primarily based on license renewals and expansions entered into recognizing the intellectual property territory licensing fee (the “IP Territory Licensing Fee”) and they are expected to generate future ongoing intellectual property usage fees (the “IP Usage Fees”) and increases in such IP Usage Fees.
During the year ended August 31, 2020, our revenues were derived within the following categories: $232,909 (2019 $198,000) of licensing revenue and $151,634 (2019 $24,610) in product and other revenues. Licensing revenues generally deliver much higher gross profit margins than do product revenues.
General and Administrative
Our general and administrative expenses increased by $11,648 during the year ended August 31, 2020, which includes $1,408,103 of non-cash compensation. The increase in our general and administrative expenses was largely due to non-cash expenses related to valuation of grants for service and share based payments. Included in the total were significant reductions to Advertising, Legal fees, R&D and travel based on changes to our operations around COVID-19 and cost containment for a significant aggregate reduction of $851,625.
Interest Expense
Interest expense for the year ended August 31, 2020 was $Nil (2019 $Nil).
Consulting fees
Our consulting fees increased during the year ended August 31, 2020 due to the involvement of additional consultants, contract updates and non-cash payments for services of $1,244,472. Our executives are typically hired and compensated as consultants and costs associated with those agreements comprise the majority of our consulting fees expense and thus a portion of our Consulting Expenses category includes certain fees that might otherwise be recognized under wages and salaries.
| 44 |
| Table of Contents |
Professional Fees
Our professional fees decreased by $299,019 during fiscal 2020 primarily due to fewer patent and trademark filings, tax and contract work. We recognize certain legal fees, tax advice fees, and accounting services all as “Professional Fees.”
| Working Capital |
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
|
|
| $ |
|
| $ |
| ||
| Current assets |
|
| 1,925,961 |
|
|
| 1,818,829 |
|
| Current liabilities |
|
| (225,917 | ) |
|
| (184,507 | ) |
| Net Working Capital |
|
| 1,700,044 |
|
|
| 1,634,322 |
|
The Company’s working capital balance increase during the year ended August 31, 2020, was due to the exercises of outstanding options and two private placements that provided significant incoming funds. The Company maintained a positive and strong working capital position throughout the year.
| Cash Flows |
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
|
| $ |
|
| $ |
| |||
| Cash flows (used in) provided by operating activities |
|
| (2,663,281 | ) |
|
| (3,005,555 | ) |
| Cash flows (used in) provided by investing activities |
|
| (26,843 | ) |
|
| (769,165 | ) |
| Cash flows (used in) provided by financing activities |
|
| 2,698,726 |
|
|
| 3,332,683 |
|
| Increase (decrease) in cash |
|
| 8,602 |
|
|
| (442,036 | ) |
Operating Activities
Net cash used in operating activities was $2,663,281 for the year ended August 31, 2020 compared with cash used in operating activities of $3,005,555 during the same period in 2019. This difference was largely due to the decreased costs pertaining to advertising and promotion, patent and trademark related filings, research and development, and travel.
Investing Activities
Net cash used in investing activities was $26,843 (2019 $769,165) for the year ended August 31, 2020 and is due to the Company’s cost incurred related to its capitalized patent related applications. The reduction is primarily based on the inclusion of the new head office facility and equipment in fiscal 2019.
Financing Activities
Cash provided from financing activities was $2,698,726 during the year ended August 31, 2020 compared to $3,332,683 during the same period in 2019.
| 45 |
| Table of Contents |
Results of Operations for our Year Ended August 31, 2019 and August 31, 2018
Our net loss and comprehensive loss and the changes between those periods for the respective items are summarized as follows:
|
|
| YEAR ENDED |
|
| YEAR ENDED |
|
|
| ||||
|
|
| August 31 |
|
| August 31 |
|
|
| ||||
|
|
| 2019 |
|
| 2018 |
|
|
| ||||
|
|
| $ |
|
| $ |
|
| Change |
| |||
| Revenue |
|
| 222,610 |
|
|
| 433,287 |
|
|
| (210,677 | ) |
| General and administrative |
|
| 4,358,130 |
|
|
| 7,017,289 |
|
|
| (2,659,159 | ) |
| Consulting fees & Employees |
|
| 1,777,934 |
|
|
| 5,332,398 |
|
|
| (3,554,464 | ) |
| Legal and professional |
|
| 670,863 |
|
|
| 289,062 |
|
|
| 381,801 |
|
| Net Loss |
|
| (4,158,413 | ) |
|
| (6,609,186 | ) |
|
| 2,450,773 |
|
Revenue
Licensing revenues of $198,000 represent the majority of revenues during the year ended August 31, 2019 and reflect delays in usage fee revenues from existing licensees in Canada waiting for approval from Health Canada on products, and other licensees initiating or ramping up their production. Revenue was primarily based on new licence agreements entered into recognizing the IP Territory Licensing Fee, and existing licenses generating usage fees. Increasing ongoing usage fees are expected as licensees begin or ramp up products or contracted minimum requirements become due.
Two years ago, the Company had one licensee and as of August 31, 2019, we had nine licensees. The territory fees consist of IP licensing fees for the transfer of the Technology at the signing of definitive agreements for the DehydraTECH technology. The additional IP Usage Fees include payments due upon transfer of the technology and installment payments that are receivable within 12 months. We are pleased that we have signed additional licenses and are looking toward revenues increasing during fiscal 2020 with the legalization of edible products in Canada expected during October 2019 and the potential for licensee product launches early in calendar 2020 in that country. Our additional and expanded licenses in the US are anticipated to generate ongoing usage fee revenues based on contracted minimums or based on licensee sales starting during our fiscal 2020.
We have made progress in signing more corporate licensees than ever before in our corporate history, but most of these licensees are small start up companies that continue to present operational risk to us. We continue to attempt to work with larger more established companies to encourage them to adopt our technology, but the markets have been slow to adopt our technology, notwithstanding our new corporate relationship with a Fortune 500 company in the nicotine industry.
Consumer product sales remain low due to ongoing challenges in securing expansive distribution opportunities, third-party production challenges, inconsistent federal vs. state or local regulations, and payment processing changes. The Company continues to pursue more widespread distribution possibilities that have the potential to unlock more significant consumer product revenues.
During the year ended August 31, 2019, our revenues were derived within the following categories: $198,000 (2018 $415,183) of intellectual property licensing revenue and $24,610 (2018 $18,104) in product and other revenues.
As fiscal 2019 came to a close, hemp oil fortified foods, and hemp seed products continued gaining consumer acceptance and provide a reason to believe that sales could increase. In addition, legislative trends in America and in many nations around the world such as Canada and the UK are supportive of additional opportunities in the hemp-based foods and supplements sector. Those trends could support higher potential consumer product sales. Release of the ChrgD+ product was successful, but sales were limited due to ongoing payment processing issues outside of the Company’s control, and due to our not being successful in obtaining widespread retail distribution channels.
| 46 |
| Table of Contents |
For 2020, the Company expects to continue to derive the majority of its revenues from technology licensing to third parties noting that IP territory fees are recognized when new definitive license agreements occur and IP usage fees are dependent upon our licensees’ opportunity to implement the technology pursuant to applicable regulatory approvals. Canadian regulatory approval for ingestible products was originally scheduled for October 17, 2019, but there are indications that actual individual product approvals required from Health Canada may delay licensee product launches into 2020 in that country. At August 31, 2015, the Company had zero technology licensing agreements entered. By August 31, 2016, we had entered several LOI’s or definitive agreements related to technology out-licensing. During the period ended August 31, 2019, we entered into nine active licensing agreements that are expected to generate additional revenue from the payment of usage fees as the licensees’ production and sales occur. It is the Company’s view its nine granted US patents, eight granted Australian patents and one granted European patent, along with its expanding patent portfolio, is a positive step in enabling the generation of more significant revenues during fiscal 2020. As of the date hereof, the Company has entered more than 10 formal letters of intent or definitive agreements and is negotiating more.
We do not expect that all of the letters of intent into which we enter will result in definitive agreements with paying customers and cannot predict how many will. We believe that strengthening and expanding our intellectual property portfolio and conducting supportive R&D will jointly contribute to strengthening revenue prospects.
General and Administrative
Our general and administrative expenses decreased by $2,659,158 during the year ended August 31, 2019. The decrease in our general and administrative expenses was largely due to non-cash expenses related to valuation of grants for service and share-based payments required by contracts included in fiscal 2018. Increases during fiscal 2019 included expanded patent applications, R&D, IR programs and the addition of employees for a total of $1,061,125, which includes $368,115 of non-cash compensation and $58,243 increase in depreciation related to new facilities and equipment.
Interest Expense
Interest expense for the year ended August 31, 2019 was $Nil (2018 $Nil). The Company has no debt at this time other than month-to-month receivables.
Consulting fees
Our consulting fees decreased by $3,887,663 primarily due to the non-cash payments for services included in fiscal 2018. Our executives are typically consultants and costs associated with those agreements comprise a significant portion of our consulting fees expense.
Legal and Professional Fees
Our professional fees increased by $381,801 to $670,863 during the year primarily due to ongoing patent and trademark filings, consultations on licensing agreements, and other advisory services. Although we always try to minimize expenses, we consider increases in costs related to patent and trademark work to reflect positive progress in executing our business plan. We recognize certain legal fees, tax advice fees, and accounting services all as “Professional Fees.”
| 47 |
| Table of Contents |
Liquidity and Financial Condition
| Working Capital |
| August 31 |
|
| August 31 |
| ||
|
|
| 2019 |
|
| 2018 |
| ||
|
|
| $ |
|
| $ |
| ||
| Current assets |
|
| 1,818,829 |
|
|
| 2,284,051 |
|
| Current liabilities |
|
| (184,507 | ) |
|
| (43,640 | ) |
| Net Working Capital |
|
| 1,634,322 |
|
|
| 2,240,411 |
|
The Company’s working capital balance decrease during the year was limited due to exercises of outstanding options and warrants and the private placement completed during the year. The Company maintained a positive and strong working capital position throughout the year.
| Cash Flows |
| August 31 |
|
| August 31 |
| ||
|
|
| 2019 |
|
| 2018 |
| ||
|
|
| $ |
|
| $ |
| ||
| Cash flows (used in) provided by operating activities |
|
| (3,005,555 | ) |
|
| (2,517,979 | ) |
| Cash flows (used in) provided by investing activities |
|
| (769,165 | ) |
|
| (155,399 | ) |
| Cash flows (used in) provided by financing activities |
|
| 3,332,683 |
|
|
| 1,867,224 |
|
| Decrease in cash |
|
| (442,037 | ) |
|
| (806,153 | ) |
Operating Activities
Net cash used in operating activities was $3,005,555 for the year compared with cash used in operating activities of $2,517,979 during the same period in 2018. This difference was largely due to the increased costs pertaining to consulting, advertising and promotion, patent and trademark related filings, legal advisory services, new employees, research and development, and travel.
Investing Activities
Net cash used in investing activities was $769,165 (2018 $155,399) for the year due to the Company’s cost incurred related to its patent applications $122,982 and our new office space and equipment $646,183.
Financing Activities
Net cash provided from financing activities was $3,332,683 during the year ended August 31, 2019 compared to net cash provided of $1,867,224 during the same period in 2018.
| 48 |
| Table of Contents |
Going Concern
The Company’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“US GAAP”) applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has a net loss attributable to its common shareholders of $3,933,996 for the year ended August 31, 2020 (2019 $4,099,420) and at August 31, 2020 had a deficit accumulated since its inception of $27,802,198 (2019 $23,868,202). The Company has a working capital balance of $1,700,044 as at August 31, 2020 (2019 $1,634,322). The Company requires additional funds to maintain its operations and developments beyond fiscal 2020. Management’s plans in this regard are to raise equity and debt financing as required, but there is no certainty that such financing will be available or that it will be available at acceptable terms. The outcome of these matters cannot be predicted at this time.
In March 2020, the World Health Organization declared COVID-19 a global pandemic. This contagious disease outbreak and any related adverse public health developments may adversely affect workforces, economies, and financial markets globally, potentially leading to an economic downturn. It is not possible for the Company to predict the duration or magnitude of the adverse results of the outbreak and its effects on the Company’s business or results of operations at this time.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Critical Accounting Policies
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with the accounting principles generally accepted in the United States of America (US GAAP). Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management's application of accounting policies. We believe that understanding the basis and nature of the estimates and assumptions involved with the aspects of our financial statements are critical to an understanding of our financial statements as more particularly described in Note 3 to our audited annual consolidated financial statements for the fiscal year ended August 31, 2020 included in this prospectus.
| 49 |
| Table of Contents |
Our Current Business
Our business plan is currently focused on the development of strategic partnerships with licensees for our patented DehydraTECH technology in exchange for up front and/or staged licensing fees and/or royalty payments over time. We continue to investigate national and international opportunities to investigate expansions and additions to our intellectual property portfolio. We plan to perform additional human clinical investigations in late calendar 2020 and early 2021 related to enhanced DehyraTECH formulations of cannabidiol in pre- and mildly-hypertensive middle-aged subjects to gather additional information on blood pressure reduction potential. The Company also plans to conduct during calendar 2020 and 2021 evaluations of DehydraTECH’s ability to improve the oral delivery characteristics and pharmacological performance of certain anti-viral drugs. We will continue to seek beneficial acquisitions of intellectual property if and when we believe it is advisable to do so.
Our current patent portfolio includes patent family applications or grants pertaining to our method of improving bioavailability and taste, and the use of DehydraTECH as a delivery platform for a wide variety of Active Pharmaceutical Ingredients (“APIs”) including, but not limited to, fat soluble vitamins; NSAIDs pain medications; and nicotine and its analogs.
The Company hopes to reduce common but less healthy administration methods, such as smoking cigarettes as a delivery method for nicotine, by way of enabling development of safe and effective oral nicotine dosage forms through licensing arrangements with major tobacco companies. The Company is aggressively pursuing patent protection in jurisdictions around the world. The Company currently has over 50 patent applications pending worldwide, with 18 patents granted to date. Due to the complexity of pursuing patent protection, the quantity of patent applications will vary continuously as each application advances or stalls. The Company is also filing new patent applications for new discoveries that arise from the Company’s R&D programs and, due to the inherent unpredictability of scientific discovery, it is not possible to predict if or how often such new applications might be filed.
During the past fiscal year the Company experienced the following significant corporate developments with respect to its current business framework:
On November 13, 2019 the Company closed the first tranche of a non-brokered private placement financing resulting in the issuance of 51,808 units at a price of $13.50 per unit with each unit being comprised of one share of common stock and one share purchase warrant for gross proceeds of $699,410.25. The warrants are exercisable for a period of two years at an exercise price of $24.00 per share until November 13, 2020 and thereafter at a price of $36.00 per share until November 13, 2021.
On November 28, 2019 the Company closed the second tranche of a non-brokered private placement financing resulting in the issuance of 8,983 units at a price of $13.50 per unit with each unit being comprised of one share of common stock and one share purchase warrant for gross proceeds of $121,275. The warrants are exercisable for a period of two years at an exercise price of $24.00 per share until November 28, 2020 and thereafter at a price of $36.00 per share until November 28, 2021.
On March 19, 2020, the Company announced that it intended to commence a program to conduct tests to research the benefits of its DehydraTECH Technology in connection with enhancing the delivery of certain antiviral drugs.
On April 21, 2020 the Company announced the filing of a strategic new US patent application under a new patent family “Compositions and Methods for Enhanced Delivery of Antiviral Agents” to utilize its DehydraTECH process in connection with antiviral drugs for the purposes of combatting infectious disease conditions potentially including, but not limited to, COVID-19, MERS, SARS, influenza, herpes and AIDS.
| 50 |
| Table of Contents |
On May 4, 2020, the Company entered into material contracts with certain investors for the sale of 295,540 shares of common stock and warrants to purchase up to 295,540 shares of common stock for gross proceeds of $2,039,228. The warrants have a five year term and are exercisable at $10.20 per share. The financing closed in two tranches on May 6, 2020 and May 11, 2020.
On May 5, 2020 the Company terminated the agreement, entered into by its subsidiary Canpharm, to provide DehydraTECH to a California-based private company for its utilization of DehydraTECH in certain THC-based beverages, due to lack of performance by the licensee.
On or around June 18, 2020, the Company submitted a grant application to the U.S. National Institutes of Health (NIH) entitled "In vitro and in vivo animal exploratory pharmacokinetic and preliminary efficacy modelling of select orally administered antiviral compounds following DehydraTECH formulation enhancement" pursuant to their National Institute of Allergy and Infectious Diseases (NIAID) Funding Opportunity Announcement (FOA) RFA-AI-20-028 - Partnerships for Countermeasures against Select Pathogens. This grant application, which has not yet been reviewed or approved, is for funding to support the Company’s second round of planned studies related to COVID-19 treatment possibilities.
On June 24, 2020, the Company announced the results of the 2020 Annual and Special Meeting held June 23, 2020. The Company held the Meeting whereby there were 1,593,993 shares of the Company represented in person or by proxy at the meeting, constituting 53.38% of the Company’s issued share capital as at May 13, 2020, being the record date of the Meeting. The matters voted upon at the Meeting and the final voting results are set forth below:
| Matter Being Voted On | For | Against | Abstain or Withheld | Broker Non-Vote | Percent Approved By |
| To Elect Christopher Bunka as a director | 910,558 | 0 | 21,862 | 661,573 | 97.66% |
| To Elect John Docherty as a director | 910,126 | 0 | 22,294 | 661,573 | 97.61% |
| To Elect Nicholas Baxter as a director | 909,975 | 0 | 22,445 | 661,573 | 97.59% |
| To Elect Ted McKechnie as a director | 907,966 | 0 | 24,454 | 661,573 | 97.38% |
| To Elect Brian Quigley as a director | 911,272 | 0 | 21,148 | 661,573 | 97.73% |
| To Appoint Davidson & Company LLP as Auditors | 1,578,817 | 0 | 15,176 | 0 | 99.05% |
| To Approve a Reverse Stock Split on a ratio of not less than 2 current shares for one reverse stock split share and not more than 30 current shares for one reverse stock split share | 1,460,205 | 126,935 | 6,853 | 0 | 91.61% |
| To Approve an amendment to the Company’s Bylaws | 885,989 | 36,480 | 9,951 | 661,573 | 95.02% |
| To ratify the lawful actions of the directors for the past year | 902,977 | 16,597 | 12,847 | 661,573 | 96.84% |
All of the proposals were described in detail in the Company’s proxy statement filed with the SEC via Edgar and with the BCSC and Ontario Securities Commission via SEDAR on May 25, 2020.
| 51 |
| Table of Contents |
On June 29, 2020, the Board approved the issuance of 11,574 restricted shares of common stock to a consultant bearing a deemed aggregate value of $100,000 or $8.64 per share as partial compensation for investor relations services to be provided to the Company. In addition, the Company has also agreed to pay the consultant a cash fee of $7,500 per month for such services and may, at its sole discretion, engage the consultant to provide additional services at additional costs.
On July 21, 2020, the Company announced filing an application with a national securities exchange in the United States to request an uplisting of the Company’s common stock.
On July 28, 2020, the Company announced that it has received ethics board approval by a European university research hospital to conduct an exploratory clinical study using cannabidiol (“CBD”) formulated together with its patented DehydraTECH™ technology to assess blood pressure reduction potential in volunteers with pre- or mildly- hypertensive, middle-aged volunteers.
On August 31, 2020, the Company announced a research and development (“R&D”) framework agreement with British American Tobacco (Investments) Limited (“BAT”) to investigate the Company’s technology for potential use in nicotine products. R&D work under the Agreement will be paid for by BAT.
On December 2, 2020, the Company announced that its DehydraTECH technology significantly improved delivery in study animals of representative drugs from two classes of antiviral therapies (a Protease Inhibitor and a Reverse Transcriptase Inhibitor) under investigation against SARS-CoV-2/COVID-19 and already in use against HIV/AIDS. The study animals were not infected with or treated for any diseases. These are the first two of a series of antiviral drugs to be tested using Lexaria’s DehydraTECH technology.
| Drug | Drug Class | AUClast* Delivery & Improvement (hr∙ng/mL) | Control (hr∙ng/mL) | AUC∞** Delivery & Improvement (hr∙ng/mL) | Control (hr∙ng/mL) |
|
Darunavir |
Protease Inhibitor |
721 ± 332 54% (p=0.036) |
469 ± 252 |
726 ± 211 35% (p=0.062) |
536 ± 223 |
|
Efavirenz |
Non-nucleoside Reverse Transcriptase Inhibitor |
752 ± 203 16% (p=0.11) |
650 ± 148 |
1072 ±40 42% (p=0.028) |
757 ±103 |
During the past fiscal year the Company experienced the following significant corporate developments with respect to its historical business framework:
On September 17, 2019, the Company announced the publication of the final study results in the peer reviewed medical journal, “Advances in Therapy” of its 2018 human clinical study evaluating CBD delivery and effectiveness using its patented DehydraTECH powered TurboCBD capsules available on the PubMed.gov website with the identification of PMID: 31512143. Advances in Therapy focuses on clinical medicine and pharmaceutical research and has been published continually since 1984.
The study was conducted and well tolerated in 12 healthy young male athletes and accordingly, an additional study to assess blood pressure reduction potential in middle-aged volunteers with pre- or mild-hypertension has been designed which in July 2020 received ethics board approval by a European university research hospital.
On or around October 21, 2019, the Company submitted an amendment to its Health Canada research license, which was originally granted on August 9, 2019, to allow for human organoleptic sensory testing. The amendment to the licence was approved by Health Canada on June 8, 2020 and remains effective until August 9, 2023.
On January 14, 2020, the Company announced that it had entered into a definitive 10-year agreement, via its subsidiary Lexaria Hemp Corp., with Boldt Runners Corporation (dba Cannadips) to provide DehydraTECH on an exclusive basis in the U.S. for use in oral pouches containing CBD.
On March 4, 2020, the Company announced that it had amended its license agreement with Universal Hemp LLC, a B2B manufacturing company of hemp-derived bulk ingredients to remove the exclusivity rights originally associated with the license and to reduce the aggregate minimum performance fees from $3,750,000 to $132,500.
| 52 |
| Table of Contents |
On November 18, 2020 the Company’s subsidiary CanPharm entered into an agreement to sell its license to use and sublicense DehydraTECH for use in the production of consumer products containing over 0.3% THC to Hill Street, with such sale closed on December 9, 2020. Accordingly, the Company’s expanded relationship with the Cannadips brand, as originally announced on January 22, 2020, by way of a definitive 10-year license agreement, to provide the Company’s patented Technology on an exclusive basis in the U.S. for use in oral pouches containing over 0.3% THC has been assigned to Hill Street.
On November 18, 2020, the Company announced that it had entered into a definitive 10-year agreement, via its subsidiary Lexaria Hemp Corp., with Hill Street to provide DehydraTECH on a non-exclusive global basis for use in multiple CBD-infused products. This agreement replaces a previous license agreement and joint venture agreement between Lexaria Hemp Corp. and Hill Street as originally announced on July 23, 2019.
The Company experienced the following significant corporate developments subsequent to August 31, 2020
On September 22, 2020, the Company announced that U.S. Patent No. 10,756,180 was granted. This patent protects the use of its DehydraTECH technology together with cannabinoids, nicotine, nonsteroidal anti-inflammatory drugs, or vitamins in mix and serve beverage formats. The patent is entitled “Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof”.
Impact of COVID-19
The emergence of COVID-19 beginning in January of 2020, now in over 220 countries and territories around the world, presents significant and unforecastable new risks to the Company and its business plan. Restrictions on national and international travel, and required business closures, have made it increasingly difficult to carry out normal business activities related to corporate finance efforts, to the pursuit of new customers, and to retail customers throughout North America who might otherwise access the products of our business partners and licensees. As a result, the COVID-19 pandemic will almost certainly increase risks of lower revenues and higher losses. We are monitoring our licensees and are working with them, where possible, to prevent default and contract terminations. In some cases, we have issued termination of contract notices in accordance to provisions within our contracts.
As a result of COVID-19, the Company is encountering significant challenges in executing its business plan and normal business operations and does not have sufficient resources to withstand a protracted term during which most business activities are curtailed. In addition, we have implemented cost containment initiatives to reduce operating expenses and preserve cash such as the dismissal of one employee, termination of contracts with two consultants and reduction of compensation payable to certain other consultants. The Company currently has six (6) employees and/or independent contractors who dedicate all or a majority of their time to the business of the Company and eight (8) consultants. We may need to dismiss additional employees or terminate services contracts to preserve resources. We have not had to close operations or locations as our contractors and staff can work remotely and our third-party facilities continue to operate. To date, we have not directly had to quarantine contractors or staff, however we have implemented additional safety precautions and measures for their protection. Due to our historic and current geographic diversity of our contractors and employees, we have long established and ongoing experience in remote work and collaboration. Our procedures and controls have been built over time to address remote working requirements.
We have not experienced any significant impacts on our material supply chains but have noted increased timelines from some third-party research facilities regarding their ability to conduct research and testing. To date, this has not significantly impacted our R&D programs, but we cannot predict whether our R&D programs will be impacted in the future.
| 53 |
| Table of Contents |
On March 19, 2020, the Company announced that it intended to commence a program to conduct tests to research the benefits of DehydraTECH in connection with enhancing the delivery of certain antiviral drugs.
The tests are intended to include a pilot human pharmacokinetic exploratory study in healthy volunteers of three antiviral drugs that had previously been studied against other coronavirus strains, comparing DehydraTECH formulations to controls without the Company’s Technology. Subsequent to March 19, 2020, the Internal Review Board (“IRB”) of one of the Universities where we submitted a study design and plan to, advised us to limit the study to two of the original antiviral drugs. Based on the requirements of the IRB, we have modified the study to two antiviral drugs. IRB approval has since been received, however, such approval is conditioned on further government regulatory approval. The Company is currently in the process of pursuing the necessary steps to file for study approval from federal regulators to conduct the study.
In parallel, the Company launched a separate rodent antiviral study to evaluate pharmacokinetic benefits from the use of DehydraTECH in the delivery of representative drugs from two classes of antiviral drugs under investigation for treatment of COVID-19. The results of that animal study were released on December 1, 2020 whereby the DehydraTECH enhanced antiviral drug formulations demonstrated increased delivery of the antiviral drugs into the bloodstream of the animals. The results of this animal study have encouraged the Company to conduct expanded investigations into antiviral drug delivery enhancement, with such investigations including remdesivir (a nucleotide reverse transcriptase inhibitor); as well as three additional drugs known to target the main protease associated with SARS-CoV-2 infection. The Company intends to make its research results available to researchers throughout the world looking to maximize the delivery of their own drug investigations. The Company’s business model relies on performing early stage studies like these to help support its efforts to form commercial relationships with more established companies.
The Company continues to monitor governmental programs being released to assist with the COVID-19 pandemic. To date, we have received a CDN$40,000 Canada Emergency Business Account (“CEBA”) for our subsidiary Kelowna Management Services Corp. (“KMSC”) with 0% interest and no principal payments required until December 31, 2022, after which the account is converted to a 3 year term loan at 5% annual interest paid monthly. CDN$10,000 is forgivable if the account is paid back CDN$30,000 after December 31, 2020 and prior to December 31, 2022. We have also received $30,732 (CDN$42,076) from the Canada Emergency Wage Subsidy (“CEWS”) program for the employees in our subsidiary KMSC that reduced costs therein.
Science and Technology
The Company is a biotechnology, oral, topical and drug delivery R&D company focused on developing and out licensing DehydraTECH for improved consumer experiences, rapidity, and delivery of bioactive compounds in oral and topical products. The Company is focusing its capital and management time on its pursuit of intellectual property, technology licensing opportunities, and an expanding portfolio of patent pending applications.
In 2014, the Company acquired the intellectual property that formed our first patent application that was filed in the same year. From that first patent application, due to ongoing R&D investigation and work by Company management, we now have over 50 patent applications pending around the world, with 18 patents granted. All of our applications and granted patents relate to DehydraTECH and certain characteristics of oral ingredient and drug delivery. Additional early stage investigation has been conducted of topically-administered products such as patches, creams and lotions.
| 54 |
| Table of Contents |
The Company developed a variety of demonstration products throughout 2015 to demonstrate the potential uses for DehydraTECH to both consumers and potential licensees. Seven (7) flavors of teas, hot chocolate, coffee, and two (2) flavors of protein energy bars were produced – all intended to utilize DehydraTECH for more palatable and efficient delivery of bioactive molecules. The Company subsequently developed additional demonstration products including powder filled capsules and mix and serve powders for beverage incorporation also intended to utilize DehydraTECH for more palatable and efficient delivery of bioactive molecules. The Company gained extensive experience and knowledge from the formulation and production of these demonstration products that facilitates assisting our licensees with the integration of DehydraTECH in their products.
In the production of our intermediate products for product manufacturers to use, each raw material, intermediate stage and completed product is assessed for compliance with all applicable regulations, and that the inputs and the finished products meet all applicable legal and quality standards including and as it relates to content; molds and mildews; heavy metals; and may measure additional components.
The US Federal Government, through the US Department of Health and Human Services, owns US Patent #6630507, which among other things, claims that
“Cannabinoids have been found to have antioxidant properties, unrelated to NMDA receptor antagonism. This new found property makes cannabinoids useful in the treatment and prophylaxis of wide variety of oxidation associated diseases, such as ischemic, age-related, inflammatory and autoimmune diseases. The cannabinoids are found to have particular application as neuroprotectants, for example in limiting neurological damage following ischemic insults, such as stroke and trauma, or in the treatment of neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease and HIV dementia.”
For reference, cannabinoids are compounds that affect cannabinoid receptors located on many human cells. CB1 receptors are widely found within the human brain; and CB2 receptors are found with the human immune system and have been linked to anti-inflammatory and other responses.
Despite independent scientific findings in many locations around the world, some regulatory agencies do not officially recognize that a human endocannabinoid system exists.
Over one hundred different cannabinoids have been isolated from the cannabis plant, most of which do not have psychoactive properties. One that does have psychoactive properties is THC. Endocannabinoids are produced naturally in the human body while Phyto cannabinoids are produced in several plant species, most abundantly in the cannabis plant.
CBD is one of the major Phyto cannabinoid forms of cannabinoids and is not psychoactive, often contributing more than 35% of the extracts from the cannabis plant resin. CBD occurs naturally in other plant species beyond cannabis. For example, the most widely acknowledged alternative source of Phyto cannabinoid is in the better understood Echinacea species, which is in widespread use as a dietary supplement. Most Phyto cannabinoids are virtually insoluble in water but are soluble in lipids and alcohol. The World Anti Doping Agency (“WADA”) has exempted CBD from its 2018 list of banned substances.
In the U.S., the 2018 Farm Bill permits hemp cultivation and allows the transport of hemp-derived products across state lines, within a tightly regulated framework. Primary among these, the plant must contain less than 0.3% THC, and state departments of agriculture must submit their plans to license and regulate hemp to the Secretary of the USDA, or otherwise comply with a federally-run hemp program. Legislative reform regarding CBD from hemp is continually evolving.
| 55 |
| Table of Contents |
Status of Current Operations
We have a main corporate website (www.lexariabioscience.com). Most of the Company’s revenues are generated from third party businesses either licensing the intellectual property associated with DehydraTECH for incorporation into their products or purchasing DehydraTECH infused intermediate product as a raw material for use within their own products.
On June 11, 2015, we initiated the simultaneous filing of a U.S. utility patent application and an International patent application under the Patent Cooperation Treaty (PCT) procedure, both through the U.S. Patent and Trademark Office (“USPTO”). These applications follow the Company’s 2014 and 2015 family of provisional patent application filings in the U.S. and serve two additional broad purposes:
|
| 1. | The Company is seeking protection of its intellectual property under international treaties. To this end, the Company has filed for PCT patent application protection. There are 153 countries that are signatories to the Patent Cooperation Treaty, including such major markets as Canada, China, India, much of Europe and the Middle East and Japan among others. |
|
|
|
|
|
| 2. | The Company has demonstrated that its lipid infusion technology may have applications beyond the delivery of just cannabinoids. Based on further formulation testing, the Company has included additional lipophilic molecules that may be delivered via oral administration utilizing its technology, widely encompassing three major market opportunities for the Company: Nicotine; NSAIDs; and Vitamins. |
In December 2015, the Company filed two further provisional patent applications in the U.S. These new applications served to further broaden the variety and applicability of base compounds that can be used when formulating DehydraTECH. The first of these applications identify compounds like edible starches (e.g., tapioca starch) that are commonly used in oral and pharmaceutical products today and could, therefore, serve as a base for formulating and incorporating DehydraTECH into a wide variety of products. The second of these applications identify emulsifier compounds like gum arabic that are commonly used in beverage products today in order to facilitate similar flexibility for formulating DehydraTECH in shelf-stable beverages.
On October 26, 2016, the USPTO issued U.S Patent No. 9,474,725, “Food and Beverage Compositions Infused with Lipophilic Active Agents and Methods of Use Thereof,” pertaining to our method of improving bioavailability and taste of certain cannabinoid lipophilic active agents in food products. This was the Company’s first patent granted and has a publish date of October 25, 2016 (March 2, 2017 in Australia No. 2015274698) and protects DehydraTECH for twenty years from the application’s filing date. Additional patent grants include, but are not limited to: the use of DehydraTECH as a delivery platform, “composition of matter” claims that protect the specific combination of substances intended to enable improved taste and bio absorption properties, that protect processes for making specific compositions of matter intended to enhance cannabinoid delivery utilizing DehydraTECH. Of note, the Company has received issuance of patents in its second and third patent families in Australia. This represents the first time the Company has been granted claims for use of DehydraTECH in connection with the treatment of specific diseases and medical conditions affecting humans, which the Company believes will prove to be of significance to the pharmaceutical industry sector as it further develops and grows. Our portfolio consists of the following granted patents:
| 56 |
| Table of Contents |
| Issued Patent # | Patent Issuance Date | Patent Family |
| US 9,474,725 B1 | 10/25/2016 | Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof
|
| US 9,839,612 B2 | 12/12/2017 | |
| US 9,972,680 B2 | 05/15/2018 | |
| US 9,974,739 B2 | 05/22/2018 | |
| US 10,084,044 B2 | 09/25/2018 | |
| US 10,103,225 B2 | 10/16/2018 | |
| US 10,381,440 | 08/13/2019 | |
| US 10,374,036 | 08/06/2019 | |
| US 10,756,180 | 08/25/2020 | |
| AU 2015274698 | 03/02/2017 | |
| AU 2017203054 | 05/17/2018 | |
| AU 2018202562 | 05/17/2018 | |
| AU 2018202583 | 05/17/2018 | |
| AU 2018202584 | 09/27/2018 | |
| AU 2018220067 | 04/18/2019 | |
| EP 3164141 | 11/11/2020 | |
| AU 2016367036 | 04/18/2019 | Methods for Formulating Orally Ingestible Compositions Comprising Lipophilic Active Agents |
| AU 2016367037 | 05/02/2019 | Stable Ready-to-Drink Beverage Compositions Comprising Lipophilic Active Agents |
The Company does not know and cannot know whether these strategies will be successful, or if successful, how long it will take to gain customer loyalty. It can be a challenge to be successful by introducing new consumer products utilizing DehydraTECH to a competitive retail marketplace, and we can offer no assurances that our licensees’ products will be commercially successful. To date, the Company has not realized significant revenues from its licensees or from the production of its products.
International Patent Protection
The Company first began work in the fields of enhanced delivery of active ingredients and drugs in 2014 focusing our efforts on R&D within the U.S. and Canadian marketplaces with our demonstration products to licence DehydraTECH to product manufacturers. Our pursuit and development of our technology has expanded our potential area of impact, both geographically and by sector. Because of the applicability of DehydraTECH to many market sectors across the globe, we have taken the necessary steps to protect that intellectual property within global markets in sectors such as cannabinoids, nicotine, vitamins, and pharmaceuticals.
Additional Molecules
The Company does not intend to create or produce consumer products ourselves, rather, its business plan is to encourage existing participants within these sectors to license and utilize DehydraTECH to enable enhanced performance of their products.
ANTIVIRALS. Viruses and bacteria cause the most common infectious diseases in the world today. Vaccines can offer protection against contracting viral and bacterial infections, whereas antiviral drugs and antibiotics respectively are required as treatments to combat disease if vaccination or other protective measures are inadequate or are not available. Early research findings have shown that some known antiviral drugs like remdesivir, interferon beta-1b, lopinavir, ritonavir and ribavirin among others, evaluated alone and in combination treatment regimens, may have utility against COVID-19 caused by infection with COVID-19. Most of the antiviral drugs currently available are used to treat infections caused by HIV, herpes viruses, hepatitis B and C viruses, and influenza A and B viruses, and are therefore being repurposed to evaluate prospective utility against COVID-19. While a host of antiviral drugs exist or are under development today as treatments for COVID-19 and other infectious disease conditions, many of them are hindered by poor water solubility which, in turn, results in their poor absorption and uptake by the body if taken orally, frequently limiting their overall therapeutic effectiveness. To attempt to overcome this, oral antiviral medications often have to be given at high doses which can result in a variety of unwanted side effects including diarrhea, headache, nausea, vomiting, stomach upset, drowsiness, dizziness, vision changes, difficulty breathing and other bodily dysfunctions. Alternatively, in some cases it is necessary to administer antiviral medications by way of needle injection for easier access to the bloodstream circumventing the gastrointestinal absorption limitations as is the case with, for instance, remdesivir, as mentioned above. However, injectable administration requires involvement of a medical practitioner which may not be easily accessible for the masses, usually increases cost of a medicine and often means that the product format isn’t as stable or requires special storage and handling considerations relative to oral medications.
| 57 |
| Table of Contents |
NICOTINE. More than 99% of all nicotine consumed worldwide is delivered through smoking cigarettes. Approximately 6,000,000 deaths per year, worldwide, are attributed primarily to the delivery of nicotine through the act of smoking according to the Centers for Disease Control and Prevention, which also estimates that over $170 billion per year is spent just in the U.S. on direct medical care costs for adult smokers. 69% of U.S. adult smokers want to quit smoking and 43% of U.S. adult smokers have attempted to quit in any twelve-month period.
Worldwide, legal retail cigarette sales were worth US$814 billion in 2018 with illegal sales thought to represent another 11.2% of the global market (bat.com) with over 5.3 trillion cigarettes sold to more than 1 billion smokers. The Company’s technology is not applicable to the combusted cigarette market: instead, it is hoped to be adopted as an alternative to combusted cigarettes.
NON-STEROIDAL ANTI-INFLAMMATORIES. NSAIDs are the second-largest category of pain management treatment options in the world and are used both for pain management and for treatment of inflammation. The anti-inflammatory therapeutic market is expected to generate $106.1 billion in 2020, globally (alliedmarketresearch.com). Incurable inflammatory autoimmune diseases included arthritis, asthma, and chronic obstructive pulmonary disease (COPD). The U.S. makes up over one-half of the global market. The opioids market (such as morphine) form the largest single pain management sector but are known to be associated with serious dependence and tolerance issues.
Some of the most commonly known NSAIDs are ASA (Aspirin), Ibuprofen (Advil, Motrin), and Acetaminophen (Tylenol - Acetaminophen is not accepted by all persons to be an NSAID). Although NSAIDs are generally a safe and effective treatment method for pain, they have been associated with a number of gastrointestinal problems including dyspepsia and gastric bleeding and certain adverse effects on human kidneys.
VITAMINS. The global vitamin and supplement market is worth $68 billion according to Euromonitor. The category is both broad and deep, comprised of many popular and some lesser known substances. Vitamins in general are thought to be an $8.5 billion annual market in the U.S. The U.S. is the largest single national market in the world, and China and Japan are the 2nd and 3rd largest vitamin markets.
Vitamin E is fat soluble and can be incorporated into cell membranes which can protect them from oxidative damage. Global consumption of natural source Vitamin E was 10,900 metric tons in 2013 worth $611.9 million.
On August 11, 2015, the Company signed a license agreement with PoViva Tea LLC for $10,000, granting the Company a 35-year non-exclusive worldwide license to unencumbered use of PoViva Tea LLC’s IP Rights, including rights of resale of Poviva Tea LLC’s DehydraTECH enhanced ViPova Teas. This license agreement ensures the Company has full access to the underlying infusion technology. On January 14, 2019, this agreement was updated whereby Poviva Corp. (formerly PoViva Tea LLC) granted the Company an exclusive license to use DehydraTECH for a period of time ending 25 years after the date of the last patent granted to Poviva Corp. We are not currently planning to continue production of ViPova Teas or any other previously manufactured DehydraTECH enhanced consumer products.
| 58 |
| Table of Contents |
Scientific testing and validation
On August 24, 2015, the Company announced achievements in enhanced gastro-intestinal absorption of CBD utilizing DehydraTECH. The third-party testing was conducted in two phases of in vitro tests beginning in June and completed in August 2015.
The independent laboratory results delivered average CBD permeability of 499% of baseline permeability, compared to CBD permeability without DehydraTECH, exceeding Company expectations. This was assessed in a strictly controlled, in vitro experiment using a human intestinal tissue model.
The tests also showed 325% of baseline gastro-intestinal permeability of CBD comparing the Company’s CBD-fortified ViPova black tea to a second control of CBD and black tea combined, without the Company’s patented formulation enhancements. This confirmed that the specialized processing undertaken by the Company during its manufacturing process together with its formulation enhancements, does indeed significantly improve preclinical absorption levels.
The bioavailability of CBD (or of THC) varies greatly by delivery method. Smoking typically delivers cannabinoids at an average bioavailability rate of 30% (Huestis (2007) Chem. Biodiverse. 4:1770–1804; McGilveray (2005) Pain Res. Manag. 10 Suppl. A:15A – 22A). By comparison, orally consumed cannabis edibles typically deliver cannabinoids at an average bioavailability rate of only 5% (Karschner et al. (2011) Clin. Chem. 57:66–75).
The Company’s findings suggested that DehydraTECH may achieve a five-fold improvement in cannabinoid absorption in edible form over that which can be achieved without its proprietary process and formulation enhancements. This conceptually supports that DehydraTECH represents a significant breakthrough in cannabinoid delivery by approximating the high absorption levels achieved as though through administration by smoking, but without the associated negative effects on human health caused by smoking.
The tests were completed in two phases culminating with testing using simulated intestinal fluid conditions that delivered these findings. These results were stronger than earlier iterations of the tests that did not use a simulated intestinal fluid environment and contributed to the Company’s understanding of the mechanisms at work. The Company believes DehydraTECH could significantly reduce individual serving requirements for CBD to consumers. This could lead to reduced costs of consumption for consumers.
The Company believes that the use of DehydraTECH to enhance the absorption of CBD in the recent laboratory tests, may be applicable to anti-viral, THC, nicotine, NSAIDs and other lipophilic compounds widely used today.
During January 2015, the Company conducted a study of nitric oxide levels in humans, as a biomarker for absorption of CBD, with the expectation that it would provide additional evidence of the efficient absorption of CBD from DehydraTECH-enhanced oral products enhanced with hemp oil, by demonstrating the elevation of nitric oxide in the human body in response to oral ingestion.
The study data from human subjects demonstrated significant elevation of systemic nitric oxide levels as a surrogate biomarker for CBD bio absorption in response to ingestion of the Company’s oral delivery. This provided clinical support for the CBD bioavailability-enhancing properties of DehydraTECH, on the premise that bioavailable CBD is known to elevate levels of the endocannabinoid anandamide in the human body which, in turn, stimulates release of nitric oxide in the vascular system.
| 59 |
| Table of Contents |
Consuming the Technology-enhanced oral products resulted in elevated levels of nitric oxide within the body. The results of the study indicated that all of Technology-enhanced oral products elicited significant increases in salivary nitric oxide, achieving levels from 110 µM to as high as 220 µM in the test subjects. The liquid oral products generally had faster initial responses in as little as 15 minutes after product ingestion, whereas the initial responses from the solid oral products required 30 minutes. The faster response time with the liquid oral products was to be expected, given the relative ease of digesting liquids versus solids. All products sustained their maximum levels of nitric oxide detection through to the 60-minute end-points used in the study, indicating a need for additional study to determine the length of time that nitric oxide levels remain elevated following production consumption.
Six healthy human subjects (3 male and 3 female) between the ages of 22 and 65 years of age were recruited for the small pilot study. Subjects were screened for cardiovascular and allergic response to hemp products, were non-smokers and did not have any history of substance or alcohol abuse. One product was studied per day across all six subjects, with each subject consuming a full product serving size. Subjects were required to refrain from eating food or using vape products for at least 12 hours before test article administration on each day of the study. Nitric oxide levels in the test subjects were assessed using a commercially available, colorimetric test kit designed to quantify systemic nitric oxide via a detectable salivary marker. Immediately before test article administration each day, all subjects were required to demonstrate a negative baseline nitric oxide saliva test. Subjects were considered to have a negative test strip reading at a level of 20 µM according to the test strip scale, and positive readings anywhere above this. Subjects performed salivary nitric oxide testing at 15, 30, 45 and 60 minutes’ post-consumption of each product. All subjects remained sedentary from baseline through to the completion of testing for each product.
In August of 2018, we released the results from our randomized, placebo-controlled, double-blind European human clinical study that evaluated TurboCBD capsules- a proprietary, DehydraTECH powered, CBD fortified hemp oil capsule developed by the Company. The degree and speed of CBD absorption into blood plasma and potential cardiovascular and cognitive performance enhancement in 12 healthy male volunteers were studied.
Key metabolic and hemodynamic performance findings linked to bioavailability enhancements were revealed in the study as released in February 2019, which compared a 90 mg dose of the Company’s DehydraTECH enhanced TurboCBD capsules to a 90 mg dose without DehydraTECH™ (the “positive control”) as well as a placebo, as follows:
|
| • | Analysis of mean arterial blood pressure (MAP) at peak blood levels of CBD achieved with the Company’s TurboCBD demonstrated a significant reduction in MAP compared to placebo (95% CI; p=0.027). This finding was not observed with the dose-matched positive control formulation for which there was no significant decrease in MAP compared to placebo (95% CI; p=0.625); |
|
| • | Cerebral perfusion was also analysed by an index of conductance in the middle cerebral artery (MCA). The findings revealed that the Company’s TurboCBD caused the greatest increase in MCA conductance relative to both the positive control formulation and placebo (95% CI; p=0.017 and P=0.002 respectively); |
Finally, over the six-hour study, analysis of the total area under the curve (AUC) demonstrated that the Company’s DehydraTECH enhanced TurboCBD capsules resulted in a notable trend for higher levels of CBD in the bloodstream overall than the positive control formulation with total AUC of 10,865 ± 6,322 observed with the Company’s formulation compared to 7,115 ± 2,978 observed with the positive control (95% CI; p=0.096). Furthermore, when normalized to body mass, the AUC at the peak CBD concentration was markedly and significantly (95% CI; p=0.02) higher with the TurboCBD 90 mg dose compared to the 90 mg dose positive control formulation.
| 60 |
| Table of Contents |
These results corroborate other in vitro and in vivo studies that evaluated DehydraTECH. Although this study evaluated absorption only of CBD and its metabolites, the Company believes nearly identical bioavailability enhancement results would be achieved with other cannabinoids.
During March of 2019 we also launched an in vivo research program to test the Company designed nanotech enhancements comprised of eleven separate animal studies and released initial results during May 2019 demonstrating measurable quantities of cannabidiol into blood in as little as 2 minutes. In each arm of the animal studies, 10 male Sprague-Dawley rats were administered CBD at 25mg per kg of bodyweight. Delivery of CBD into the bloodstream was monitored over a 60-minute duration. In the first animal study results it announced, the Company compared its standard DehydraTECH formulation that combined cannabinoids with long-chain fatty acids (“LCFA”) using the Company’s patented dehydration processing technique to a concentration-matched formulation utilizing coconut oil which is a commonly used MCT oil in the cannabis edibles industry, with the following key findings:
|
| · | At 2 minutes DehydraTECH’s LCFA formulation delivered measurable CBD in blood, compared to no measurable CBD in blood until 6 minutes and onwards for the MCT oil formulation. |
|
|
|
|
|
| · | At 15 minutes DehydraTECH’s LCFA formulation achieved a CBD blood concentration level that was 475% more than the MCT oil formulation; and, the DehydraTECH LCFA formulation CBD blood levels reached at 15 minutes were greater than the CBD blood levels reached by the MCT oil formulation at any time point during the 60-minute evaluation. |
|
|
|
|
|
| · | At 60 minutes DehydraTECH’s LCFA formulation achieved a CBD blood concentration level of 319% more than the MCT oil formulation. |
|
|
|
|
|
| · | Over the entire 60-minute study, the animals that received the standard DehydraTECH LCFA formulation achieved an average maximum CBD blood concentration level that was 334% more than the average maximum blood concentration level of the animals that received the MCT oil formulation (p<0.0021). |
|
|
|
|
|
| · | Over the entire 60-minute study, the area under the curve (AUC) (total quantity of CBD delivered) for the DehydraTECH LCFA formulation was 389% more than the MCT oil formulation (p<0.0011). |
The Company also tested for brain tissue concentrations to quantify 8-hour CBD delivery from the DehydraTECH-enabled LCFA formulation compared to the MCT oil formulation and DehydraTECH’s LCFA formulation outperformed the MCT oil formulation by 246%.
The Company released additional results from its March 2019 research program wherein animal testing showed that combining the Company’s DehydraTECH delivery technology with generic nanotech techniques delivered 1,137% more CBD into animal brain tissue following oral ingestion than certain existing industry formulations. The Company combined its DehydraTECH delivery technology with a standard form of nanotechnology and analyzed subsequent delivery into brain tissue following oral ingestion. Delivery of CBD into the brain was reported 8 hours after dosing, as follows:
|
| · | The DehydraTECH LCFA formulation without nanotech achieved an average brain tissue accumulation level that was 246% higher than the average for those animals that received the MCT oil formulation (p=0.0013). |
|
|
|
|
|
| · | The DehydraTECH LCFA formulation with nanotech achieved an average brain tissue accumulation level that was 1,137% higher than the average for those animals that received the MCT oil formulation (p=0.0178). |
| 61 |
| Table of Contents |
Further preclinical results demonstrated that Enhanced DehydraTECH led to 811% more CBD delivery into blood than generic industry MCT coconut-oil formulations (p=0.00008); and 110% more CBD into blood than DehydraTECH in its traditional format (p=0.02).
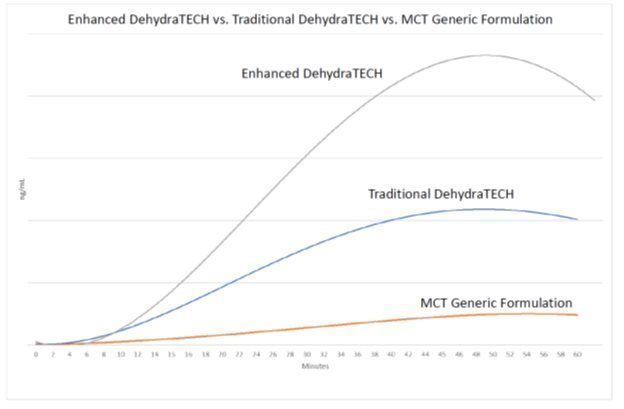
|
| · | Enhanced DehydraTECH delivered roughly twice as much CBD to animal blood at all measured time points in the study from the 15-minute mark onwards, compared to traditional DehydraTECH; and during the same time points from 717% to 1098% more CBD than the generic industry MCT coconut oil formulations. |
|
|
|
|
|
| · | Enhanced DehydraTECH delivered more CBD to blood in just 12 minutes than the MCT coconut-oil formulation was able to achieve at any point during the 1-hour test duration. |
|
|
|
|
|
| · | Enhanced DehydraTECH is even faster acting, reaching a maximum blood concentration level (“tmax”) in just 45 minutes compared to traditional DehydraTECH at 50 minutes and the MCT coconut oil formulation at 57 minutes. |
|
|
|
|
|
| · | Enhanced DehydraTECH delivered an astonishing 1,937% more CBD into animal brain tissue after 8 hours compared to generic industry MCT coconut oil formulations; and 487% more than traditional DehydraTECH. |
Both traditional DehydraTECH and Enhanced DehydraTECH delivered maximum blood concentration levels prior to the 60-minute end-of-test, with levels tapering off thereafter. The DehydraTECH technology therefore demonstrates both fast onset and fast offset as tested which is of interest for dose titration purposes when repeated dosing is desired.
We have also completed our first preclinical study evaluating DehydraTECH used in a topical cream formulation for absorption of CBD through human skin. Results proved significant increases in both speed and quantity of CBD absorption through skin when compared to control formulations. The absorption study was performed on human skin at a California-based laboratory that specializes in Franz diffusion cell skin permeability testing. DehydraTECH was used together with a sophisticated oil-in-water emulsion formulation design and compared to a series of matching oil-in-water emulsion formulations prepared with the same CBD inputs, with and without DehydraTECH and with and without two leading skin penetration enhancers currently used in the skin products industry. Several factors were measured, including the time required to detect CBD skin penetration and quantity, and peak amounts of CBD absorbed into and through the skin, at multiple testing intervals over a 48-hour duration.
| 62 |
| Table of Contents |
The Company’s DehydraTECH-enabled topical formulation, absent either of the commercial penetration enhancers, was the fastest acting for absorption into the epidermis, dermis or through the skin into the systemic fraction representing permeation into the underlying circulatory system. The Company’s DehydraTECH-enabled product also had no odor even without the use of perfumes, contrary to other cannabinoid industry products that can be quite strongly odoriferous without the use of masking perfumes.
Furthermore, the Company’s DehydraTECH-enabled topical formulation without the addition of either of the commercial penetration enhancers, demonstrated the highest overall average quantity of CBD delivered through the skin and into the representative systemic fraction of all the formulations tested, with as much as a 225% increase in CBD permeability when compared to the highest performing commercial penetration enhancer formulation assessed and almost a 1,900% increase in CBD permeability when compared to a control formulation that was devoid of both DehydraTECH or any commercial penetration enhancers. The commercial skin penetration enhancers only demonstrated performance that was on par or superior to the DehydraTECH-enabled formulations tested in so far as total CBD absorption into the shallow epidermis or dermis was concerned.
We have also completed our first ingestible nicotine in vivo (animal) absorption study. The Company is pursuing the use of DehydraTECH as a possible new nicotine delivery method, an edible dose absorbed through the gastrointestinal tract, with potential both as a nicotine replacement therapy as well as an alternative product format for regular tobacco users.
DehydraTECH delivered the following major nicotine absorption performance improvements: 1,160% faster delivery of equivalent peak quantities of nicotine to the bloodstream than achieved with controls (within 15 min vs. 2.9 hours), 148% gain in the quantity of peak nicotine delivery to the bloodstream relative to controls, 560% higher brain levels of nicotine where nicotine effects are focused, compared to controls, lower urine levels of nicotine excreted than controls, for enhanced nicotine activity and bioavailability over the course of the study, lower quantities of key liver metabolites in the bloodstream than controls as hypothesized, suggesting bypass of first pass liver metabolism.
The study was designed to principally assess the relative ingestible nicotine absorption performance of DehydraTECH-powered formulations compared to concentration-matched control formulations that lacked any form of delivery enabling technology in rats. Nicotine was administered in a nicotine polacrilex derivative format as is widely commercialized today in nicotine replacement therapy products such as chewing gums. Twelve male rats were divided into four groups of three, such that DehydraTECH and control formulations were each tested at a 1 mg/Kg and 10 mg/Kg dosage level. Formulations were administered orally and all rats were cannulated for blood collection at multiple intervals over an 8 hour duration post-dosing with the first data collection at the 15-minute mark. Urine and feces were also collected for up to a 24-hour duration post-dosing, and essential organ tissue samples were also collected for examination after the study. All samples were subjected to analytical testing in order to quantify the levels of nicotine therein, as well as the levels of three major liver metabolites thereof, hydroxycotinine, nicotine N’-oxide and cotinine, in order to assess the relative metabolite levels absorbed by the different formulations. The Company’s hypothesis was tested to prove that DehydraTECH would influence more rapid and complete intestinal bio absorption of nicotine lymphatically with less metabolic degradation by the liver. All animals were also assessed for general tolerability of the administered formulations. The study was conducted at the same independent laboratory in Philadelphia where the Company completed its initial CBD absorption study in 2015.
| 63 |
| Table of Contents |
The DehydraTECH formulations generally achieved faster absorption, higher peak absorption and higher overall quantities of nicotine, on average, in the blood than the concentration-matched control formulations at both the 1mg and 10 mg/Kg doses tested. Furthermore, as previously reported, there were no obvious signs of gastrointestinal distress such as vomiting or diarrhea indicating that the animals appeared to tolerate the treatment well.
Nicotine blood levels were evaluated multiple times over a period of 8 hours after dosing. In the 10mg/Kg dosing arm, the control formulation required nearly 3 hours to reach similar levels of blood absorption that the DehydraTECH formulation reached in only 15 minutes. Furthermore, the DehydraTECH formulation went on thereafter to demonstrate peak plasma levels that were 148% of those achieved by the control formulation. If replicated in human studies, these findings are suggestive that DehydraTECH could be more effective in elevating blood nicotine levels through edible formats much more quickly and substantially than previously theorized, potentially making ingestible nicotine preparations a viable alternative to today’s available product formats while also leading to a more rapid nicotine craving satiation.
Analysis of the liver metabolites revealed, as expected, that overall levels in the blood of two of the three metabolites studied were higher in the control group than in the DehydraTECH formulation group at the 10 mg/Kg dose. This result was especially pronounced in the 45-minute to 2-hour time interval post-dosing which is consistent with the expected timing of release of metabolites in higher quantity into the bloodstream by the liver following normal physiological processing of ingested nicotine with the control preparation, compared to DehydraTECH that is believed to elude first pass liver metabolism. The DehydraTECH formulation also demonstrated lower quantities of nicotine in the rat urine at both doses, which is consistent with the fact that the levels of nicotine in the rat blood remained higher over the duration of the study with the DehydraTECH formulation than with the control. The study also revealed that the DehydraTECH formulation at the 10 mg/Kg level achieved up to 5.6-times as much nicotine upon analysis of the rat brain tissue than was recovered with the matching control formulation. These findings together perhaps suggest prolongation of nicotine effectiveness with the DehydraTECH formulation which may also be beneficial in humans to control cravings over an extended time-period from a single edible nicotine dose.
In our follow-up third-party in vivo statistically significant study, including two groups of 20 animals, further defining delivery of nicotine in edible form at each of the 2, 4, 6, 8 and 10-minute intervals post-dosing, with 90.2% greater delivery than the concentration-matched control formulation by the 10-minute mark (95% CI; p=0.044), and significantly greater absorption levels than the control formulation at all subsequent time points in the study. Speed of onset is a key attribute for oral drug administration, and it is of particular importance for the consideration of non-inhalation nicotine delivery formats.
Key highlights of the follow-up study were as follows:
|
| · | Peak Level: 79% improvement in peak blood levels (maximum concentration or “Cmax”) at 394 ng/mL using the Company’s DehydraTECH technology vs. 220 ng/mL with the control (95% CI; p=0.0257); |
|
|
|
|
|
| · | Total Quantity: 94% improvement in total quantity of nicotine delivered (area under the curve or “AUC”) to the blood during the 60-minute course of the study, at 266 hr•ng/mL versus 137 hr•ng/mL (95% CI; p=0.0086); |
|
|
|
|
|
| · | Rapidity: the Company’s technology delivered nicotine into the blood stream by the first time interval of blood sampling at the 2-minute mark. On average, the Company’s technology delivered 203 ng/mL to the blood in aggregate of the 2, 4, 6, 8, 10, 12 and 15-minute time points, compared to only 120 ng/mL in aggregate over the same period by the control, an improvement of 70% (95% CI; p=0.0004). |
| 64 |
| Table of Contents |
In addition to the above described scientific testing and validation studies, the Company has also conducted various cannabinoid formulation experiments, together with potential DehydraTECH licensees, on chocolates, candies, gummies, mouth-melts, chocolate bars, protein bars, beverages such as beer, spices, tea, coffee, supplements and more over the past several years. Beverage formulations have produced cannabinoid water-based products including de-alcoholized beer that mask unwanted cannabis flavor and are fast acting. Chocolate formulations were reported as being the fastest acting, most consistent, and best-tasting products relative to comparator control formulations in approximately 70% of cases in a 2017 consumer study. As well, on March 22, 2016, the Company announced results from another chocolate formulation consumer study in which test subjects ranked those chocolates that had been created with DehydraTECH as the best tasting, most palatable and providing the best overall experience of the chocolates sampled. Furthermore, the test subjects in that study indicated a time of onset of the cannabis oil effects in as little as 15-20 minutes on average. The study included 12 volunteers who were all regular cannabis consumers with experience ingesting conventional edibles. All chocolates used in the study were blinded (unmarked) in order that the subjects could not discern the product formulations applied.
During March of 2020, we also announced that we were commencing a program to study the prospective benefits of our DehydraTECH drug delivery platform for enhancing delivery and effectiveness of certain antiviral drugs in the fight against COVID-19. As an initial step, the Company announced that it intends to conduct a pilot human pharmacokinetic exploratory study in healthy volunteers of three antiviral drugs that have previously been studied against other coronavirus strains, comparing DehydraTECH formulations to controls without DehydraTECH.
We intend to conduct the study at a leading Canadian university to which we have already submitted a study design and plan and from which we have already received ethics board approval. The study, however, remains subject to further government regulatory approval. The Company is currently in the process of pursuing the necessary steps to file for study approval from federal regulators to conduct the study.
In parallel, the Company launched a separate rodent antiviral study to evaluate pharmacokinetic benefits from the use of DehydraTECH in the delivery of representative drugs from two classes of antiviral drugs under investigation for treatment of COVID-19. The results of that animal study were released on December 1, 2020 whereby the DehydraTECH enhanced antiviral drug formulations demonstrated increased delivery of the antiviral drugs into the bloodstream of the animals. The results of this animal study have encouraged the Company to conduct expanded investigations into antiviral drug delivery enhancement, with such investigations including remdesivir (a nucleotide reverse transcriptase inhibitor); as well as three additional drugs known to target the main protease associated with SARS-CoV-2 infection. The Company intends to make its research results available to researchers throughout the world looking to maximize the delivery of their own drug investigations.
The Company continually focuses on new R&D programs to expand on its understanding of DehydraTECH, including (i) plans for in vitro absorption tests on Vitamin E and Ibuprofen, having received positive results from its in vitro testing on Nicotine; and (ii) plans for defining the molecular compatibility, absorption rates, timing and viable formats of delivery of DehydraTECH. In addition, the Company intends to investigate the potential of additional commercial applications for DehydraTECH. These include, but are not limited to ongoing programs to explore methods to integrate nanoemulsification chemistry techniques together with DehydraTECH and to further enhance intestinal bioabsorption rates with its technology, as well as ongoing programs to expand the types and breadth of product form factors into which DehydraTECH can be applied. Depending on how many of these tests are undertaken, R&D budgets are expected to vary significantly. It is in our best interests to remain flexible at this early stage of our R&D efforts in order to capitalize on potential novel findings from early stage tests and thus re-direct research into specific avenues that offer the most reward.
| 65 |
| Table of Contents |
Historical Business Technology out-licensing
On May 14, 2016, the Company entered into a Licensing Agreement with Nuka Enterprises, LLC (“Nuka”) for a two-year period, to utilize DehydraTECH to create, test, manufacture, and sell marijuana-infused consumable and/or topical products, in the state of Colorado, with an option of extending the terms of the Licensing Agreement to Washington, Oregon, and California. On April 30, 2018, the Company announced a new 10-year renewal licensing agreement with Nuka, maker of 1906 brand cannabis chocolates and other edible products. The new agreement provides Nuka with semi-exclusive ability to utilize the Technology across the U.S. Nuka also acquired an option to expand its products and brand to Canada, including using the Company’s existing chocolate and confections contract manufacturer licensee Cannfections Group Inc. The agreement incorporates new rights in product categories in addition to the original chocolate formats, which include candies, beverages, capsules and pills, and topical creams. On May 21, 2019, we announced a major expansion in operations by Nuka over the next two years into Illinois, Oklahoma (amended from Ohio), Massachusetts, Michigan and other states. The comprehensive semi-exclusive agreement provides Nuka and 1906 with competitive technological advantages until 2028. This license was subsequently disposed of and assigned to Hill Street with the asset disposition by CanPharm.
On January 25, 2018, the Company announced it entered a definitive technology licensing agreement with a 7-year term with Cannfections Group Inc. whereby the Company is providing its patented Technology to empower next-generation performance in cannabis infused chocolates and candies to be developed and sold in Canada and internationally. This license, with respect to products prepared with greater than 0.3% THC was subsequently disposed of and assigned to Hill Street with the asset disposition by CanPharm.
In connection with the disposition of CanPharm’s assets to Hill Street, as agreed to on November 18, 2020 with an effective closing on December 9, 2020, the historical license to utilize the Technology with THC-infused beverages and the historical joint venture agreement to produce THC consumer products that were entered into between CanPharm and Hill Street as at July 24, 2019 were effectively terminated via their assignment to Hill Street.
| 66 |
| Table of Contents |
Current Business Technology out-licensing
On January 15, 2019, the Company announced that its wholly-owned subsidiary Lexaria Nicotine and Altria Ventures Inc., an indirect wholly-owned subsidiary of Altria Group, Inc. (“Altria”), executed definitive agreements to pursue innovation in oral, reduced risk nicotine consumer products using the Company’s patented Technology. Altria was granted a license to use the Company’s Technology for oral nicotine delivery forms on an exclusive basis in the United States and a non-exclusive basis elsewhere globally. Altria will pay Lexaria Nicotine a royalty on revenue generated from the sale of nicotine products containing DehydraTECH, until such time it may acquire 100% ownership in Lexaria Nicotine. There is no requirement that Altria must acquire 100% ownership in Lexaria Nicotine. Altria has determined not to continue investing into Lexaria Nicotine and accordingly, they now retain non-exclusive provisions within their license for the U.S region.
On May 15, 2019, the Company, via its wholly-owned subsidiary, Lexaria Hemp Corp. entered into a ten-year license agreement to provide Nuka with the immediate ability to utilize DehydraTECH with CBD products across the U.S. marketplace to the extent compliant with all federal, state and local laws.
On July 10, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Hemp Corp., to provide the Company’s Technology to Nic’s Beverages Ltd. for use in CBD-based beverages to be produced and sold throughout the United States.
On July 11, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Lexaria Hemp Corp., to provide the Company’s Technology to Universal Hemp LLC, a B2B manufacturing company of hemp-derived bulk ingredients to the nutraceutical and consumer packaged goods industries to be produced and sold across the U.S. immediately, and in Canada when regulations permit. On March 4, 2020, this license was revised to remove exclusivity provisions that Universal Hemp previously enjoyed and reduce the minimum fees payable over the term of the license to $132,500. This agreement was further amended on December 8, 2020 to further reduce the minimum fees payable over the term of the license to $120,000.
On November 18, 2020, the Company, via its wholly-owned subsidiary Lexaria Hemp Corp., and Hill Street terminated and mutually released each other from the obligations and liabilities associated with a license to use DehydraTECH to enhance CBD infused beverages and a joint venture agreement to produce CBD-infused products. As part of the termination of these agreements, Lexaria Hemp Corp. agreed to enter into a new license agreement with Hill Street, entitling Hill Street to use DehydraTECH with multiple product lines that incorporate CBD globally on a non-exclusive basis.
| 67 |
| Table of Contents |
Following the disposition of the CanPharm assets, the Company will retain 5 licensees from its hemp and nicotine business divisions.
The continuation of our business interests in these sectors is dependent upon obtaining further financing, a successful program of development, and, ultimately, achieving a profitable level of operations. The issuance of additional equity securities by us could result in a significant dilution in the equity interests of our current stockholders. Obtaining commercial loans, assuming those loans would be available, will increase our liabilities and future cash commitments.
We are not yet profitable and have not yet demonstrated our ability to generate significant revenues from our business plan. We will require additional corporate funds if our existing capital is not sufficient to support the Company until potential future profitability is reached. There are no assurances that we will be able to obtain further funds required for our long-term operations. We expect to require additional operating capital during our fiscal 2021 year. There can be no assurance that additional financing will be available to us when needed or, if available, that it can be obtained on commercially reasonable terms. If we are not able to obtain the additional financing on a timely basis, we will be unable to conduct our operations as planned, and we will not be able to meet our other longer-term obligations as they become due. In such event, we could be forced to scale down or perhaps even cease our operations. There is uncertainty as to whether we can obtain additional long-term financing if we do in fact require it.
Our business plan anticipates that we will hire two to four additional staff members during fiscal 2021 to enhance operations in our office and federally-licensed laboratory space. However, the effects of the COVID-19 pandemic call into question our ability to hire additional staff. We expect to be able to utilize contracted third-parties for our R&D testing programs, instead focusing our capital on higher value-added aspects of our research and development, and scientific test planning.
Our Company relies on the business experience of our existing management, on the technical abilities of consulting experts, and on the technical and operational abilities of its operating partner companies to evaluate business opportunities.
Competition
The biopharmaceutical industry is characterized by intense competition and rapid innovation. Our competitors may be able to develop other drug delivery platforms that are able to achieve similar or better results than DehydraTECH. Our potential competitors include major multinational pharmaceutical companies, established biotechnology companies, specialty pharmaceutical companies, universities and other research institutions. Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations and well-established sales forces. Smaller or early stage companies may also prove to be significant competitors, particularly as they develop novel approaches to oral or topical drug delivery that our DehydraTECH is also focused on. Established pharmaceutical companies may also invest heavily to accelerate discovery and development of novel therapeutics or to in-license novel therapeutics that could make the product candidates that can be delivered using DehydraTECH obsolete. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors, either alone or with collaborative partners, may succeed in developing, acquiring or licensing API delivery technologies that are more effective, safer, more easily commercialized or less costly than our DehydraTECH proprietary technology or secure patent protection that we may need for the enhancement of our DehydraTECH. We believe the key competitive factors that will affect the development and commercial success of any DehydraTECH enhanced product candidates are efficacy, safety, tolerability, reliability, convenience of use, price and reimbursement. We face competition from segments of the pharmaceutical, biotechnology and other related markets that pursue the development of API delivery platforms which may be more effective or cost efficient than our DehydraTECH. We anticipate that we will continue to face intense and increasing competition as new advanced API delivery technologies become available. There can be no assurance that our competitors are not currently developing, or will not in the future develop, technology that is equally or more effective or is more economically attractive than any of our current or any enhanced versions of DehydraTECH.
| 68 |
| Table of Contents |
Competition in alternative health sectors and in consumer products in the U.S. is fierce. We expect to encounter competitive threats from existing participants in the sector and new entrants with competing technologies. Although PoViva Corp. has filed patent applications to protect intellectual property, there is no assurance that patents beyond those already issued will be granted nor that other firms may not file superior patents pending. Food supplements, organic foods, and health food markets are all well established and the Company and/or its licensees will face many challenges within these markets. The Company is also aware of various competing technologies that exist in the marketplace that claim to also enhance the bio absorption of bioactive molecules as the Company has demonstrated through repeated in vitro and in vivo scientific testing with DehydraTECH. By and large, these technologies are mostly forms of nanotechnology that generally claim to enable the formation of microencapsulated microemulsions of active ingredients. These technologies can enable exceptional water solubility of ingredients and can impart improved intestinal bio absorption as a result, but do not necessarily offer the breadth of performance and value enhancing benefits that the Company’s DehydraTECH technology offers to its licensees.
Competition in nicotine, alternative nicotine delivery and nicotine cessation sectors in the U.S. is comprised of long-established entities, brands, and new technologies competing to create less harmful options. The sectors are complicated by the significant historical empirical data of older products or technologies versus the more limited published supporting data regarding the effects of new products or technologies. Due to the size of the sectors we expect to encounter competitive threats from existing participants and unknown new entrants. There is no assurance that other technologies already deployed, or in development, will not form the basis of product formats that competitors or consumers choose to utilize. It is also possible that historic delivery methods that have been in use and the familiarity with them may prevent adoption of products utilizing DehydraTECH in alternative delivery formats. Competing technologies or products may utilize known delivery formats or entirely new and unforecastable formats. The Company has demonstrated through scientific testing that DehydraTECH delivers nicotine rapidly and effectively through oral delivery. We believe that if we can educate and influence consumers to adopt a food-grade edible product format, and if US regulatory bodies authorize such formats, we may be able to offer a competitively successful new product format that utilizes DehydraTECH.
The Company believes that DehydraTECH offers a host of benefits beyond what competing technologies can offer, including superior oral palatability, a more appealing and all-natural ingredient compositional profile from an oral product and beverage formulation perspective, more predictable time of delivery into bloodstream and certain target tissues, and superior scalability and cost effectiveness from a manufacturing perspective. The Company believes that DehydraTECH is, therefore, significantly distinguished from competing technologies in these respects, and has a view of growing the breadth and number of licensees that will adopt DehydraTECH into their product offerings going forward. The Company believes that these competitive advantages together with its wealth of scientific data showing noteworthy bio absorption enhancements with DehydraTECH constitute a compelling value proposition for its prospective licensees, and it intends to continue to pursue license arrangements in the multiple bioactive ingredient sectors identified in its issued and pending patent applications.
| 69 |
| Table of Contents |
Compliance with Government Regulation
The U.S. Farm Bill, which passed in December 2018, and the ambiguity regarding the incorporation of CBD into ingested and topical products has had significant impacts on the industry segments that we operate and have products in and potentially changes some of the regulatory compliance risks that may affect our business. The bill includes lifting restrictions on advertising, marketing, banking and other financial services as well as allowing interstate commerce for hemp and hemp-derived CBD, removing barriers for intellectual property protections under federal law such as patents and trademarks, as well as several other measures that may positively impact these industry segments overall. The impact the Bill may have on other regulatory bodies and their regulations will require ongoing monitoring to determine the outcome and timing of any revisions.
DehydraTECH may also have applications in completely separate sectors such as vitamins, NSAIDs, and nicotine. We have no products nor operations in any of these sectors today, although we have commenced formulation development for research and validation purposes in each of these areas. We have a formal relationship with the largest cigarette company in the U.S., the Altria Group, and have conducted R&D with that company related to the possible development of nicotine oral products. We do not know whether the Altria Group will utilize DehydraTECH within any oral nicotine product category although they do possess a license to do so. If we enter any of these sectors at any time, we will be exposed to and of necessity will have to comply with, all local, state and federal regulations in each of those sectors. As a result of the possibility of the Company being involved in a number of disparate business sectors, compliance with government regulations could require significant resources and expertise from our Company.
Contractors and Employees
We utilize employees, sub-contractors and consultants for the company’s intellectual property development and licensing, and business operations. We have three full-time employees (including one executive officer) and may add research personnel during the next 12-month period to expand our internal R&D capacity. None of our employees is represented by a labor union and we consider our employee relations to be good. We primarily engage with independent contractors to serve our executive needs.
The Company has an agreement with CAB Financial Services Ltd., wholly-owned by Christopher Bunka, for a 3-year term management contract as Chief Executive Officer effective January 1, 2019. The annual compensation payable is CDN$350,000 per year.
The Company appointed John Docherty as President of the Company effective April 15, 2015. The Company compensates Mr. Docherty by way of an employment agreement and an agreement with Docherty Management Limited, wholly-owned by John Docherty with annual compensation of CAD$300,000 for a 3-year term effective January 1, 2019.
Both of the Chief Executive Officer and the President of the Company are entitled to the following performance incentives:
| 70 |
| Table of Contents |
Performance Incentives
|
| · | A performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the Board. Compensation equal to 2% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. Certain compensation to be paid upon a change of control excluding certain circumstances and participation in the Company’s approved stock option plans. |
On June 1, 2018, the Company executed an updated three-year consulting contract with M&E Services Ltd. (M&E), a company wholly-owned by Mr. Spissinger, with monthly compensation of CAD$12,000 including an 8% annual increase. The Company may pay Mr. Spissinger a bonus from time to time, at its sole discretion. Mr. Spissinger will be entitled to receive additional common stock-based and stock option-based bonuses upon achieving certain milestones during the time of his consultancy with the Company.
|
| · | Compensation equal to 1% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. |
Our business plan contemplates increases in the number of employees and other personnel over the next 12-month period to enhance operational, sales and our in-house R&D capacity dependent upon adequate funding. When beneficial to do so, we will continue to outsource contract employment or engagements as needed. It is not possible to accurately project potential needs into the future based on circumstances that may or may not occur.
Research and Development
The Company incurred $387,074 for the year ended August 31, 2020 and $555,730 for the year ended August 31, 2019 in research and development expenditures during the period ending August 31, 2020. Specific R&D programs are in ongoing development and will be tightly related to our financial ability to undertake each research phase for each API. Due to our expanding portfolio coverage, we are continuing to examine accelerated timetable options for testing, research and development.
The Company’s in vitro absorption test of DehydraTECH enhanced nicotine molecules and its in vivo absorption tests on DehydraTECH enhanced CBD molecules yielded positive results. Ongoing testing plans are proceeding to (i) conduct in vitro absorption tests with DehydraTECH enhanced ibuprofen; and (ii) further define molecular compatibility, absorption rates, timing and viable formats of delivery.
| 71 |
| Table of Contents |
The Company continually focuses on new R&D programs to investigate potential additional commercial applications for the incorporation of DehydraTECH. These include, but are not limited to, ongoing programs to explore methods to integrate nanoemulsification chemistry techniques together with DehydraTECH that have demonstrated positive results to date, programs to further enhance intestinal bio absorption rates with DehydraTECH, as well as ongoing programs to expand the types and breadth of product form factors into which DehydraTECH can be applied. Depending on how many of these tests are undertaken, R&D budgets are expected to vary significantly. It is in our best interests to remain flexible at this early stage of our R&D efforts in order to capitalize on potential novel findings from early stage tests and thus re-direct research into specific avenues that offer the most reward.
Subsidiaries
The Company has its wholly-owned subsidiaries Lexaria CanPharm ULC, Lexaria CanPharm Holding Corp., PoViva Corp., Lexaria Hemp Corp., Kelowna Management Services Corp. and Lexaria Pharmaceutical Corp., and our majority owned subsidiary Lexaria Nicotine LLC. On January 15, 2019, the Company announced the initial investment of $1,000,000 from Altria Ventures Inc., an indirect wholly-owned subsidiary of Altria Group, Inc., for a 16.667% equity interest along with certain other rights in Lexaria Nicotine LLC.
Legal Proceedings
We know of no material, existing or pending legal proceedings against our Company, nor are we involved as a plaintiff in any other material proceeding or pending litigation. There are no other proceedings in which any of our directors, executive officers or affiliates, or any registered or beneficial stockholder, is an adverse party or has a material interest adverse to our interest.
| 72 |
| Table of Contents |
All directors of our Company hold office until the next annual meeting of the security holders or until their successors have been elected and qualified. The officers of our Company are appointed by our Board and hold office until their death, resignation or removal from office. Our directors, director nominee, and executive officers and their ages, positions held, and duration as such, are as follows:
| Name |
| Position Held with our Company |
| Age |
| Date first Elected or Appointed |
| Christopher Bunka |
| Chairman, Chief Executive Officer, and Director |
| 59 |
| Oct. 26, 2006 |
| John Docherty |
| President and Director |
| 50 |
| April 15, 2015 |
| Allan Spissinger |
| Chief Financial Officer |
| 51 |
| May 31, 2017 |
| Nicholas Baxter |
| Director |
| 67 |
| July 8, 2011 |
| Ted McKechnie |
| Director |
| 73 |
| Sept. 16, 2015 |
| Brian Quigley |
| Director |
| 47 |
| Aug. 14, 2019 |
| Al Rees e Jr. |
| Director Nominee |
| 71 |
| N/A |
Business Experience
The following is a brief account of the education and business experience of each director and executive officer during the past five years, indicating each person's principal occupation during the period, and the name and principal business of the organization by which he was employed.
Christopher Bunka – Chairman, Chief Executive Officer and Director
Mr. Bunka has been Chairman of the Board and CEO since 2006 and was primarily responsible for the corporate pivot from older business activities to bioscience. Mr. Bunka is a serial entrepreneur and has been involved in several private and public companies since the late 1980’s. He was well known for more than a decade as a part-time business commentator in print and radio, as well as an author. He has extensive experience in the capital markets, corporate governance, project acquisition and corporate finance. He is a named inventor on some of the Company’s pending patent applications.
Since 1988, Mr. Bunka has been the CEO of CAB Financial Services Ltd., a private holding company located in Kelowna, Canada. He is a venture capitalist and corporate consultant.
John Docherty – President and Director
Mr. Docherty was appointed President of the Company effective April 15, 2015. Prior to joining the Company, Mr. Docherty was former President and Chief Operating Officer of Helix BioPharma Corp. (TSX: HBP), where he led the company’s pharmaceutical development programs for its plant and recombinantly derived therapeutic protein product candidates.
Mr. Docherty is a senior operations and management executive with over 20 years experience in the pharmaceutical and biopharmaceutical sectors. He has worked with large multinational companies and emerging, private and publicly held start-ups. At Helix, Mr. Docherty was also instrumental in the areas of investor/stakeholder relations, capital raising, capital markets development, strategic partnering, regulatory authority interactions and media relations, and he also served as a management member of its board of directors. Prior to this, Mr. Docherty was President and a board member of PharmaDerm Laboratories Ltd., a Canadian drug delivery company that developed unique microencapsulation formulation technologies for use with a range of active compounds.
| 73 |
| Table of Contents |
Mr. Docherty has also held positions with companies such as Astra Pharma Inc., Nu-Pharm Inc. and Price Water house Coopers’ former global pharmaceutical industry consulting practice. He is a named inventor on issued patents and pending patent applications and he has a M.Sc. in pharmacology and a B.Sc. in Toxicology from the University of Toronto.
He has served as a director of the Company since April 29, 2016.
Allan Spissinger – Chief Financial Officer
Prior to concentrating on finance and accounting, Mr. Spissinger worked within the Informational Technologies (IT) sector for over a decade; specializing in corporate IT infrastructure and software development projects. Mr. Spissinger joined the audit and assurance department at PricewaterhouseCoopers (PwC) where he obtained his Chartered Professional Accountant (CPA) designation focusing on financial reporting and Sarbanes-Oxley (SOX) compliance in the following sectors: resources, manufacturing and technologies. Mr. Spissinger joined the Company in September 2014 as a corporate controller. His positive mentorship, excellent communication and extensive leadership skills have enabled him to successfully manage a variety of private businesses for over 20 years.
Nicholas Baxter - Director
Mr. Baxter was appointed as a member of the Board of Lexaria Corp. in 2009. Mr. Baxter received a Bachelor of Science (Honours) from the University of Liverpool in 1975, and has worked on oil & gas projects in many areas of the world. Since the 1980’s, he has worked with companies in the public markets both in the U.K. and in Canada. Mr. Baxter brings extensive real-world experience as a board member.
Mr. Baxter serves as an independent member on the Company’s Audit and Finance Committee, its Compensation Committee and its Corporate Governance and Nominating Committee.
Ted McKechnie – Director
Mr. McKechnie is a well-recognized thought leader in the Canadian food industry. In the past, Mr. McKechnie was president of Maple Leaf Foods, an owner and senior executive at Humpty Dumpty and a senior leader at Pepsi Co. After a distinguished career as an executive and marketer specializing in food manufacturing, he now focuses on moving the Canadian food sector into the future. Besides being the chairman of Food Starter’s board, Mr. McKechnie is also the Chairman/CEO of The Davies Group and William Davies Consulting Inc. Mr. McKechnie is also a chairman of the board for Advanced Technology For Food Manufacturing, serves on the Board Of Governors for St Jeromes University.
Mr. McKechnie is often called upon by think tanks, the government and industry leaders to offer insights on how to grow the food sector and add more value to the Canadian economy.
Mr. McKechnie serves as an independent member on the Company’s Audit and Finance Committee and its Compensation Committee.
Brian Quigley – Director
Mr. Quigley has been a senior Consumer Packaged Goods executive for over 20 years of Brand Building, Marketing, Operations, Leadership and General Management experience leading business transformations that deliver shareholder returns for public and private equity investors. Mr. Quigley is one of the founders of Green Sky Strategy. Before founding Green Sky, he spent 16 years at the Altria Group, with 7 years as President and CEO for U.S. Smokeless Tobacco and Nu-Mark, Altria’s innovation Company. In his time at Altria, Brian spearheaded the companies Harm Reduction strategies and worked to deliver results by creating change in the U.S. Tobacco business. Prior to Altria, Brian held branding and leadership roles with several companies, including Pinnacle Foods Corporation, International Home Foods, which is now part of ConAgra, Inc., and in the advertising industry. Brian has launched dozens of new products, created consumer focused innovation strategies and built businesses and cultures that deliver results. Brian is motivated by helping to change lives with meaningful brands.
Mr. Quigley serves as an independent member on the Company’s Compensation Committee and its Corporate Governance and Nominating Committee.
Al Rees e Jr. – Director Nominee
Mr. Rees e has agreed to join the Board upon the closing of this offering. Mr. Reese has over 40 years experience in public and private businesses and has served in b oard positions for energy companies and commercial banks. He was the Chief Financial Officer of a multi-billion public energy company and has directed over 50 acquisitions and financings ranging in size from several hundred thousand dollars to multibillion dollars. Mr. Reese was CFO of ATP Oil and Gas from 1999 until 2014 during which time he guided ATP in equity, debt, and mezzanine transaction totaling over $10 billion. Mr. Reese received his Bachelor’s Degree in Business Administration (Finance) from Texas A&M University in 1971 and his Master of Business Administration from University of Houston in 1977, and is a Certified Public Accountant.
Our Board has determined that Mr. Rees e is independent within the meaning of the Nasdaq Rule 5605(a)(2). Upon the closing of this offering, Mr. Rees e will be appointed to the Company’s Board and to the Company’s A udit and F inance committee, as its financial expert, and to the Company’s C orporate G overnance and N ominating C ommittee .
| 74 |
| Table of Contents |
Family Relationships
There are no family relationships among any of our directors or officers.
Involvement in Certain Legal Proceedings
None of our directors, executive officers, promoters or control persons has been involved in any of the following events during the past five years:
|
| 1) | A petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing; |
|
|
|
|
|
| 2) | A conviction in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses); |
|
|
|
|
|
| 3) | The subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities: |
|
| i. | Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity |
|
|
|
|
|
| ii. | Engaging in any type of business practice; or |
|
|
|
|
|
| iii. | Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws; |
|
| 4) | Such person was the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in paragraph (f)(3)(i) of this section, or to be associated with persons engaged in any such activity; |
|
|
|
|
|
| 5) | Found by a court of competent jurisdiction in a civil action or by the SEC to have violated any Federal or State securities law, and the judgment in such civil action or finding by the SEC has not been subsequently reversed, suspended, or vacated; |
|
|
|
|
|
| 6) | Found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated; |
| 75 |
| Table of Contents |
|
| 7) | The subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of: |
|
| i. | Any Federal or State securities or commodities law or regulation; or |
|
|
|
|
|
| ii. | Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or |
|
|
|
|
|
| iii. | Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
|
| 8) | The subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Code of Ethics
We adopted a Code of Ethics applicable to our officers, directors and employees. If we make any amendments to our Code of Ethics other than technical, administrative, or other non-substantive amendments, or grant any waivers, including implicit waivers, from a provision of our Code of Ethics to our Chief Executive Officer, Chief Financial Officer, or certain other finance executives, we will disclose the nature of the amendment or waiver, its effective date and to whom it applies in a Current Report on Form 8-K filed with the SEC.
Board and Committee Meetings
Our Board held four formal meetings and several informal meetings during the year ended August 31, 2020. All proceedings of the Board taken at a formal meeting were evidenced by minutes taken at such meeting. All other matters approved by the B oard outside of any formal meeting were evidenced by resolutions consented to by all the directors. Such resolutions consented to in writing by the directors entitled to vote on that resolution at a meeting of the directors are, according to the Nevada Revised Statutes and our Bylaws, as valid and effective as if they had been passed at a meeting of the directors duly called and held.
| 76 |
| Table of Contents |
Corporate Governance and Nominating Committee
At the closing of this offering, our Corporate Governance and Nominating Committee will consist of Nicholas Baxter, Brian Quigley and Al Reese Jr. The functions of the Corporate Governance and Nominating Committee will include, in part:
|
| · | identifying and recommending candidates for membership on our Board; |
|
| · | reviewing and recommending the composition of our committees; |
|
| · | making recommendations to our Board concerning governance matters; |
|
| · | establish an orientation and continuing education program for newly elected Board members; and |
|
| · | assessing the effectiveness of the Board as a whole. |
Audit and Finance Committee and Audit Committee Financial Expert
Our Board has determined that , upon the closing of this offering, Chris topher Bunka shall be removed from our Audit and Finance Committee and replaced by Al Rees e Jr .
Currently , our Audit and Finance Committee consists of Christopher Bunka, Ted McKechnie and Nicholas Baxter.
Our Board has determined that Al Rees e qualifies as an "audit committee financial expert" as defined in Item 407(d)(5)(ii) of Regulation S-K, and is "independent" as the term is used in Item 7(d)(3)(iv) of Schedule 14A under the Securities Exchange Act of 1934, as amended.
We believe that the members of our Audit and Finance Committee are collectively capable of analyzing and evaluating our consolidated financial statements and understanding internal controls and procedures for financial reporting.
Compensation Committee
On July 2, 2020, the Board appointed a Compensation Committee comprised of the following initial members: Ted McKechnie, Nicholas Baxter and Brian Quigley, all being independent directors of the B oard. A Compensation Committee charter was adopted by the Board to govern the Compensation Committee.
Executive Compensation
The particulars of the compensation paid to the following persons:
|
| a) | our principal executive officer; |
| 77 |
| Table of Contents |
|
| b) | each of our two most highly compensated executive officers who were serving as executive officers at the end of the years ended August 31, 2020 and August 31, 2019; and |
|
|
|
|
|
| c) | up to two additional individuals for whom disclosure would have been provided under (b) but for the fact that the individual was not serving as our executive officer at the end of the years ended August 31, 2020 and August 31, 2019, |
who we will collectively refer to as the named executive officers of our Company, are set out in the following summary compensation table, except that no disclosure is provided for any named executive officer, other than our principal executive officer, whose total compensation did not exceed $100,000 for the respective fiscal year:
SUMMARY COMPENSATION TABLE
| Name and Principal Position |
| Year |
| Salary ($) |
|
| Bonus ($) |
|
| Stock Awards ($) |
|
| Option Awards ($) |
|
| Non-Equity Incentive Plan Compensation ($) |
|
| Non-Qualified Deferred Compensation Earnings ($) |
|
| All Other Compensation ($) |
|
| Total ($) |
| ||||||||
| Christopher Bunka(1), |
| 2020(4) |
| - |
|
| - |
|
| - |
|
| 153,065 |
|
| - |
|
| - |
|
| 300,802 |
|
| 453,867 |
| ||||||||
| Chairman, Chief Executive Officer & Director |
| 2019 |
| - |
|
|
| - |
|
| - |
|
|
| - |
|
| - |
|
|
| - |
|
|
| 223,280 |
|
| 223,280 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| John Docherty(2) |
| 2020(4) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 275,614 |
|
|
| - |
|
|
| - |
|
|
| 242,521 |
|
|
| 518,135 |
|
| President & Director |
| 2019 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 195,740 |
|
|
| 195,740 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Allan Spissinger(3) |
| 2020(4) |
|
| - |
|
|
| - |
|
|
| - |
|
| 143,886 |
|
|
| - |
|
|
| - |
|
|
| 121,664 |
|
|
| 265,550 |
| |
| Chief Financial Officer |
| 2019 |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
| - |
|
| 112,377 |
|
| 112,377 |
| ||||
|
| (1) | Mr. Bunka was appointed as Chairman, President, Chief Executive Officer, and director on October 26, 2006, and was Chief Financial Officer of our company from April 29, 2016 to May 31 2017. He resigned as President on April 15, 2015. We pay Mr. Bunka a consulting fee through CAB Financial Services Ltd., where he is also the Chief Executive Officer. |
|
|
|
|
|
| (2) | Mr. Docherty became President on April 15, 2015 and a director on April 29, 2016. We pay Mr. Docherty a consulting fee through his wholly-owned company Docherty Management Ltd. |
|
|
|
|
|
| (3) | Mr. Spissinger became Interim Chief Financial Officer on June 1, 2017 and Chief Financial Officer June 1, 2018. We pay Mr. Spissinger a consulting fee through his wholly-owned company M&E Services Ltd. |
|
|
|
|
|
| (4) | The fair value of the stock options awarded was estimated using the Black-Scholes option pricing model with the following assumptions: expected volatility of 96%; risk-free interest rate of 0.35%; expected life of 5 years; and dividend yield of 0%. |
Our Company is currently paying consulting fees to our Chief Executive Officer CAD$29,706 per month, our President CAD$25,609 per month and our Chief Financial Officer CAD$13,997 per month.
Consulting Agreements
The Company has negotiated a 3-year term renewal management contract with Chief Executive Officer Christopher Bunka effective January 1, 2019. The annual compensation payable is CDN$350,000 per year.
The Company appointed John Docherty as President of the Company effective April 15, 2015. The Company had an agreement with Docherty Management Limited, solely owned by John Docherty with compensation of CAD$180,000 plus applicable taxes per year and has negotiated a 3-year term renewal management contract CAD$300,000 per year.
| 78 |
| Table of Contents |
The contracts for the services of the Chief Executive Officer and President of the Company also include the following performance incentives:
A performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the Board. Compensation equal to 2% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. Certain compensation to be paid upon a change of control excluding certain circumstances and participation in the Company’s approved stock option plans.
On June 1, 2018, the Company executed a thirty-six month contract with M&E Services Ltd., a wholly-owned company by Allan Spissinger, as Chief Financial Officer with monthly compensation of CAD$12,000 plus applicable taxes, including an annual 8% increase plus applicable taxes. Mr. Spissinger is also entitled to an incentive of compensation equal to 1% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances.
Other than as set out in this registration statement on Form S-1, we have not entered into any employment or consulting agreements with any of our current officers, directors or employees.
Grants of Plan-Based Awards Table
During the fiscal year ended August 31, 2020, we issued the following plan based awards to our named executive officers:
| Compensation Securities | |||||||||||||||||||
| Name and position |
| Type of compensation security |
| Number of compensation securities, number of underlying securities, and percentage of class |
|
| Date of issue or grant |
| Issue, conversion or exercise price ($) |
|
| Closing price of security or underlying security on date of grant ($) |
| Closing price of security or underlying security at year end ($) |
| Expiry date | |||
| Christopher Bunka CEO |
| Stock Options |
|
| 23,333 |
|
| 04/23/2020 |
|
| 10.20 |
|
|
|
|
|
| 04/23/2025 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| John Docherty President |
| Stock Options |
|
| 18,333 |
|
| 02/07/2020 |
|
| 14.10 |
|
|
|
|
|
| 02/07/2025 | |
|
|
|
|
|
| 13,333 |
|
| 04/23/2020 |
|
| 9.60 |
|
|
|
|
|
| 04/23/2025 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Allan Spissinger CFO |
| Stock options |
|
| 21,666 |
|
| 04/23/2020 |
|
| 9.60 |
|
|
|
|
|
| 04/23/2025 | |
| 79 |
| Table of Contents |
Outstanding Equity Awards at Fiscal Year End
The particulars of unexercised options, stock that has not vested and equity incentive plan awards for our named executive officers are set out in the following table:
| OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | ||||||||||||||||||||||||||||||||||
|
|
| OPTION AWARDS |
| STOCK AWARDS |
| |||||||||||||||||||||||||||||
| Name |
| Number of Securities Underlying Exercisable (#) |
|
| Number of Securities Underlying Unexercised Options Unexercisable (#) |
|
| Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
|
| Option Exercise Price |
|
| Option Expiration Date |
| Number of Shares or Units of Stock That Have Not Vested (#) |
|
| Market Value of Shares or Units of Stock That Have Not Vested ($) |
|
| Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
|
| Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
| ||||||||
| Christopher Bunka |
|
| 23,333 |
|
|
| - |
|
|
| - |
|
| $ | 10.20 |
|
| 2025/04/23 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| John Docherty |
|
| 10,000 |
|
|
| - |
|
|
| - |
|
| $ | 3.30 |
|
| 2021/04/15 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
| 18,333 |
|
|
| - |
|
|
| - |
|
| $ | 14.10 |
|
| 2025/02/07 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
| 13,333 |
|
|
| - |
|
|
| - |
|
| $ | 9.60 |
|
| 2025/04/23 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Allan Spissinger |
|
| 21,666 |
|
|
| - |
|
|
| - |
|
| $ | 9.60 |
|
| 2025/4/23 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
Option Exercises
During our fiscal year ended August 31, 2020, no named executive officer exercised any options.
| 80 |
| Table of Contents |
Compensation of Directors
As of January 2019, we implemented agreements for compensating our directors for their services in their capacity as directors for CAD$30,000 per year paid quarterly in advance. As of August 31, 2020, three of our Directors are accepting compensation for their services.
During the year ended August 31, 2020, an aggregate of 13,333 stock options were granted to three of our directors with an exercise price of $9.60 expiring April 23, 2025, valued at $88,544 and included in consulting expense replacing cancelled options.
Pension, Retirement or Similar Benefit Plans
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We have no material bonus or profit sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that stock options may be granted at the discretion of the Board or a committee thereof.
Indebtedness of Directors, Senior Officers, Executive Officers and Other Management
None of our directors or executive officers or any associate or affiliate of our Company during the last two fiscal years is or has been indebted to our Company by way of guarantee, support agreement, letter of credit or other similar agreement or understanding currently outstanding.
Compensation Committee Interlocks and Insider Participation
During the majority of the fiscal year ended August 31, 2020, we did not have a Compensation Committee or another committee of the board of directors performing equivalent functions. Instead, the entire Board performed the function of Compensation Committee . Our Board approved the executive and director compensation updates, with the entire board acting as the Compensation Committee. Updated compensation is as disclosed in this registration statement on Form S-1. On July 2, 2020, the Board established a Compensation Committee comprised of the following independent directors: Ted McKechnie, Nicholas Baxter and Brian Quigley.
Compensation Committee Report
As the Compensation Committee was recently formed, it did not, during the fiscal year ended August 31, 2020, hold any meetings and therefore it has not prepared a compensation committee report. The Compensation Committee Charter , as adopted by the Board to govern the Compensation Committee, is available at its website.
| 81 |
| Table of Contents |
PRINCIPAL STOCKHOLDERS
The following table sets forth information as of January 4, 2021 regarding the beneficial ownership of our common stock by (i) those persons who are known to us to be the beneficial owner(s) of more than 5% of our common stock, (ii) each of our directors, director nominees and named executive officers, and (iii) all of our directors, director nominees and executive officers as a group. Except as otherwise indicated, the beneficial owners listed in the table below possess the sole voting and dispositive power in regard to such shares and have an address of c/o Lexaria Bioscience Corp. #100 – 740 McCurdy Road, Kelowna, British Columbia V1X 2P7. As of January 4, 2021 there were 3,001,477 shares of our common stock outstanding on a post-reverse split basis.
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of our common stock subject to options, warrants, notes or other conversion privileges currently exercisable or convertible, or exercisable within 60 days of the date of this table, are deemed outstanding for computing the percentage of the person holding such option, warrant, note, or other convertible instrument but are not deemed outstanding for computing the percentage of any other person. Where more than one person has a beneficial ownership interest in the same shares, the sharing of beneficial ownership of these shares is designated in the footnotes to this table.
| Name and Address of Beneficial Owner |
| Amount and Nature of Beneficial Ownership |
|
| Percentage of Class |
| ||
| Christopher Bunka; Kelowna BC Canada |
|
| 483,455 | (1) |
|
| 16.11 | % |
| Nicholas Baxter; Aberdeenshire, UK* |
|
| 16,000 | (2) |
|
| 0.53 | % |
| John Docherty; Toronto, Ontario |
|
| 95,742 | (3) |
|
| 3.19 | % |
| Ted McKechnie; Toronto, Ontario* |
|
| 18,191 | (4) |
|
| 0.61 | % |
| Allan Spissinger; Langley, BC* |
|
| 25,639 | (5) |
|
| 0.85 | % |
| Brian Quigley; Richmond, VA* |
|
| 3,333 | (6) |
|
| 0.11 | % |
| Al Rees e Jr. * |
|
| 917 |
|
|
| 0.03 | % |
| Directors , Director Nominees and Executive Officers as a Group ( 7 persons) |
|
| 635,843 |
|
|
| 21.43 | % |
*Less than 1%
(1) Includes 215,911 shares held in the name of C.A.B. Financial Services and 237,543 shares held directly by Christopher Bunka, chairman, chief executive officer and a director of our Company. Includes 23,333 options which are exercisable at $10.20 and 6,667 warrants exercisable at $10.50.
(2) Includes 5,000 options exercisable at $9.60.
(3) Includes 18,333 options which are exercisable at $14.10, 10,000 options which are exercisable at $3.30, and 13,333 options exercisable at $9.60. John Docherty is the President and a Director of our Company
(4) Includes 5,000 options exercisable at $9.60.
(5) Includes 21,667 options exercisable at $9.60. Allan Spissinger is chief financial officer of our Company.
(6) Includes 3,333 options exercisable at $9.60.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
No director, executive officer, shareholder holding at least 5% of shares of our common stock, or any family member thereof, had any material interest, direct or indirect, in any transaction, or proposed transaction since the beginning of the years ended August 31, 2020 and 2019, in which the amount involved in the transaction exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at the year end for the last three completed fiscal years.
| 82 |
| Table of Contents |
We have entered into an underwriting agreement, dated , 2021, with H.C. Wainwright & Co., LLC (“Wainwright” or the “representative”) as the representative of the underwriters named below. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each underwriter named below, and each underwriter named below has, severally and not jointly, agreed to purchase the number of shares of common stock, pre-funded warrants and warrants listed next to its name in the following table at the public offering price, less the underwriting discounts and commissions, set forth on the cover page of this prospectus:
| Underwriter |
| Number of |
|
| Number of |
|
| Number of |
| |||
| H.C. Wainwright & Co., LLC |
|
|
|
|
|
|
|
| ||||
| Total |
|
|
|
|
|
|
|
|
|
|
|
|
The underwriters are offering the shares of common stock, pre-funded warrants and warrants subject to their acceptance of the shares of common stock, pre-funded warrants and warrants from us and subject to prior sale. The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the shares of common stock, pre-funded warrants and warrants offered by this prospectus are subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the shares of common stock, pre-funded warrants and warrants if any such securities are taken. However, the underwriters are not required to take or pay for the securities covered by the underwriters’ option described below.
Option to Purchase additional Securities
We have granted the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to an aggregate of 159,999 additional shares of common stock at the public offering price per share, less underwriting discounts and commissions, and/or additional warrants to purchase up to 159,999 shares of common stock at the public offering price per warrants, less underwriting discounts and commissions, in any combination thereof.
Discounts, Commissions and Expenses
The underwriters have advised us that they propose to offer the shares of common stock, the pre-funded warrants and the warrants to the public at the combined public offering prices set forth on the cover page of this prospectus and to certain dealers at those prices less a concession not in excess of $ share or pre-funded warrant, as applicable, and related warrants. After this offering, the combined public offering prices and concession to dealers may be changed by the underwriters. No such change will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The shares of common stock, pre-funded warrants and warrants are offered by the underwriters as stated herein, subject to receipt and acceptance by them and subject to their right to reject any order in whole or in part. The underwriters have informed us that they do not intend to confirm sales to any accounts over which they exercise discretionary authority.
| 83 |
| Table of Contents |
|
|
| Per |
|
| Per |
|
| Total Without |
|
| Total With Full |
| ||||
| Public offering price |
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
| Underwriting discounts and commissions (8%) |
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
The underwriters propose to offer the shares, pre-funded warrants and warrants to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriters may offer some of the shares and warrants, and, if applicable, pre-funded warrants and warrants, to other securities dealers at such price less a concession not in excess of $ per share and warrant. If all of the shares, pre-funded warrants and warrants offered by us are not sold at the public offering price, the representative may change the offering price and other selling terms by means of a supplement to this prospectus.
We have also agreed to pay certain expenses of the representative relating to the offering, including: (a) a management fee equal to 1.0% of the gross proceeds raised in the offering; (b) $50,000 for non-accountable expenses; (c) up to $100,000 for fees and expenses of legal counsel and other out-of-pocket expenses; and (d) up to $12,900 for clearing costs.
We estimate that the total expenses of the offering payable by us, excluding the total underwriting discount, will be approximately $417,000.
Representative’s Warrants
We have also agreed to issue to the representative or its designees, at the closing of this offering, warrants (the “Representative’s Warrants”) to purchase a number of shares of common stock equal to 8% of the number of shares of common stock sold in the offering, including any shares of common stock underlying the pre-funded warrants sold in the offering and including shares issued upon any exercise of the underwriters’ option to purchase additional securities. The Representative’s Warrants will be in the same form as the warrants issued to the investors in this offering, except that the Representative’s Warrants will have an exercise price of $___ (equal to 125% of the public offering price per share) and will have a termination date of the five year anniversary of the commencement of the sales pursuant to this offering. Pursuant to FINRA Rule 5110(e), the Representative’s Warrants and any shares of common stock issued upon exercise of the Representative’s Warrants shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of commencement of sales of this offering, except the transfer of any security: (i) by operation of law or by reason of reorganization of the issuer; (ii) to any FINRA member firm participating in the offering and the officers, partners, registered persons or affiliates thereof, if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; (iii) if the aggregate amount of our securities held by the Representative or related persons does not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity in the fund; (v) the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth above for the remainder of the time period; (vi) if we meet the registration requirements of Forms S-3, F-3 or F-10; or (vii) back to us in a transaction exempt from registration with the SEC . The Representative’s Warrants and the shares of common stock underlying the Representative’s Warrants are registered on the registration statement of which this prospectus forms a part.
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
| 84 |
| Table of Contents |
Lock-Up Agreements
We and each of our directors and officers have agreed not to offer, sell, agree to sell, directly or indirectly, or otherwise dispose of any shares of common stock or any securities convertible into or exchangeable for shares of common stock for a period of 90 days after the closing date of the offering pursuant to the underwriting agreement without the prior written consent of Wainwright. These lock-up agreements provide for limited exceptions and their restrictions may be waived at any time by Wainwright. In addition, subject to an exception after 90 days after the closing date of the offering, we have agreed to not issue any securities that are subject to a price reset based on the trading prices of our common stock or upon a specified or contingent event in the future, or enter into any agreement to issue securities at a future determined price, for 12 months following the date of closing of this offering, which prohibition may be waived at any time by Wainwright.
Right of First Refusal
We have granted the representative a right of first refusal, for a period of 18 months from the consummation of this offering, to act as sole book-running manager, sole underwriter or sole placement agent, for each and every future public and private offering or capital-raising transaction of equity, equity-linked or debt securities of the Company or subsidiary of the Company, on terms and conditions customary for such transaction.
We shall pay the representative the cash and warrant compensation provided above on the gross proceeds provided to us by investors that participated in this offering or were introduced to us by the representative or which we met during our engagement of the representative in any public or private offering or capital-raising transaction within nine months following the expiration or termination of our engagement of the representative.
Indemnification
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters or such other indemnified parties may be required to make in respect thereof.
Nasdaq Listing
We are in the process of applying to have our shares of common stock and warrants listed on Nasdaq under the symbols “LEXX” and “LEXXW”. There is no established public trading market for the warrants and pre-funded warrants, and we do not expect a market to develop for the pre-funded warrants. There is no assurance that a market will develop for the warrants. In addition, we do not intend to apply to list the pre-funded warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants will be limited.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us, and should not be relied upon by investors.
| 85 |
| Table of Contents |
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering, the underwriters may engage in stabilizing transactions, overallotment transactions, syndicate covering transactions and penalty bids in connection with our common stock.
Stabilizing transactions permit bids to purchase shares of common stock so long as the stabilizing bids do not exceed a specified maximum.
Overallotment transactions involve sales by the underwriters of shares of common stock in excess of the number of shares the underwriter is obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriter is not greater than the number of shares that it may purchase in the option to purchase additional shares. In a naked short position, the number of shares involved is greater than the number of shares in the option to purchase additional shares. The underwriter may close out any short position by exercising its option to purchase additional shares and/or purchasing shares in the open market.
Syndicate covering transactions involve purchases of common stock in the open market after the distribution has been completed in order to cover syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering.
Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on Nasdaq, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
| 86 |
| Table of Contents |
In connection with this offering, the underwriters also may engage in passive market making transactions in our common stock in accordance with Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market maker’s bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Other Relationships
Certain of the underwriters and their affiliates have in the past and may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which they have received or may in the future receive customary fees.
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
| 87 |
| Table of Contents |
The rights of our stockholders are be governed by Nevada law, Articles of Incorporation and Bylaws, as amended. The following briefly summarizes the material terms of our common stock and preferred stock. We urge you to read the applicable provisions of Nevada Corporation Law, our Articles of Incorporation and our Bylaws.
Authorized Capital Stock
Our authorized capital stock consists of 220,000,000 shares of common stock, par value $0.001 per share. As of August 31, 2020, there were 3,001,477 shares of our common stock outstanding.
Common Stock
We are authorized to issue up to a total of 220,000,000 shares of common stock, par value $0.001 per share. Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of our stockholders. Holders of our common stock have no cumulative voting rights. Further, holders of our common stock have no preemptive or conversion rights or other subscription rights. Upon our liquidation, dissolution or winding-up, holders of our common stock are entitled to share in all assets remaining after payment of all liabilities and the liquidation preferences of any of our outstanding shares of preferred stock. Subject to preferences that may be applicable to any outstanding shares of preferred stock, holders of our common stock are entitled to receive dividends, if any, as may be declared from time to time by our Board out of our assets which are legally available. Such dividends, if any, are payable in cash, in property or in shares of capital stock.
The holders of shares of our common stock entitled to cast at least a majority of the total votes entitled to be cast by the holders of all of our outstanding capital stock, present in person or by proxy, are necessary to constitute a quorum at any meeting. If a quorum is present, an action by stockholders entitled to vote on a matter is approved if the number of votes cast in favor of the action exceeds the number of votes cast in opposition to the action. The vote of a majority of our stock held by shareholders present in person or represented by proxy and entitled to vote at the Meeting will be sufficient to elect Directors or to approve a proposal.
Pre-Funded Warrants
The following summary of certain terms and provisions of the pre-funded warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration and Exercise Price
Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.001. The pre-funded warrants will be immediately exercisable and will not expire prior to exercise. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock. The pre-funded warrants will be issued in certificated form.
| 88 |
| Table of Contents |
Exercisability
The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon notice from the holder to us, the holder may increase or decrease the beneficial ownership limitation in the holder’s pre-funded warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants provided that any increase in the beneficial ownership limitation shall not be effective until 61 days following notice to us.
Cashless Exercise
If, at the time a holder exercises its pre-funded warrants, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Transferability
Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the pre-funded warrants. Rather, the number of shares of common stock to be issued will be rounded up to the nearest whole number.
Trading Market
There is no established public trading market for the pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants will be limited.
Right as a Stockholder
Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our common stock with respect to the shares of common stock underlying the pre-funded warrants, including any voting rights, until they exercise their pre-funded warrants. The pre-funded warrants will provide that holders have the right to participate in distributions or dividends paid on our common stock.
Fundamental Transaction
In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction.
| 89 |
| Table of Contents |
Warrants
The following summary of certain terms and provisions of the warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of warrant for a complete description of the terms and conditions of the warrants. The warrants will be issued in book-entry form and will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC pursuant to a warrant agency agreement between us and Computershare, Inc. together with its wholly-owned subsidiary, Computershare Trust Company, N.A., as warrant agent .
Duration and Exercise Price
The warrants are exercisable from and after the date of their issuance and expire on the five year anniversary of such date, at an exercise price per share of common stock equal to $_____. The warrants will be governed by the terms of a global warrant held in book-entry form. The holder of a warrant will not be deemed a holder of our underlying common stock until the warrant is exercised. No fractional shares of common stock will be issued in connection with the exercise of a warrant. Instead, for any such fractional share that would have otherwise been issued upon exercise of warrants, we will round such fraction up to the next whole share.
Exercisability
The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon notice from the holder to us, the holder may increase or decrease the beneficial ownership limitation in the holder’s pre-funded warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants, provided that any increase in the beneficial ownership limitation shall not be effective until 61 days following notice to us.
Cashless Exercise
If, at the time a holder exercises its warrants, a registration statement registering the issuance of the shares of common stock underlying the warrants under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the warrants.
Transferability
Subject to applicable laws, a warrant may be transferred at the option of the holder upon surrender of the warrant to us together with the appropriate instruments of transfer.
| 90 |
| Table of Contents |
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the warrants. Rather, the number of shares of common stock to be issued will be rounded to the nearest whole number.
Trading Market
There is currently no established public trading market for the warrants, and there is no assurance that a market will develop. We are in the process of applying to have the warrants listed on Nasdaq under the symbol “LEXXW”. We will not consummate this offering unless our shares of common stock and warrants are approved for listing on Nasdaq. We have not applied to list the warrants on the CSE. After this offering, our common stock will continue to trade on the CSE.
Right as a Stockholder
Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the warrants do not have the rights or privileges of holders of our common stock with respect to the shares of common stock underlying the warrants, including any voting rights, until they exercise their warrants. The warrants will provide that holders have the right to participate in distributions or dividends paid on our common stock.
Fundamental Transaction
In the event of a fundamental transaction, as described in the warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the warrants will be entitled to receive upon exercise of the warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction. Additionally, as more fully described in the warrants, in the event of a fundamental transaction (as defined in the warrants), the holders of the warrants will be entitled to receive consideration in an amount in cash equal to the Black Scholes value of the warrants determined according to a formula set forth in the warrants, provided, however, that, if the fundamental transaction is not within our control, including not approved by our Board, then the holder shall only be entitled to receive the same type or form of consideration (and in the same proportion), at the Black Scholes value of the unexercised portion of the warrant, that is being offered and paid to the holders of our common stock in connection with the fundamental transaction.
Warrant Agent
The warrants will be issued pursuant to the terms of a warrant agency agreement between us and Computershare, Inc. together with its wholly-owned subsidiary, Computershare Trust Company, N.A. , as warrant agent. The warrant agent may resign upon 30 days’ written notice to us and our transfer agent , if applicable . We have the right to remove the warrant agent upon 30 days’ prior written notice to the warrant agent, our transfer agent and the holders of any warrant certificates. If the warrant agent resigns or is removed, we will appoint a successor warrant agent. If we do not do so within 30 days, then any holder of a warrant certificate may petition a court of competent jurisdiction to appoint a successor warrant agent and we will be deemed to be the warrant agent pending such appointment. In the warrant agency agreement, we have agreed to indemnify the warrant agent against certain liabilities.
Anti-Takeover Provisions of Nevada State Law
Certain anti-takeover provisions of Nevada law could have the effect of delaying or preventing a third-party from acquiring us, even if the acquisition arguably could benefit our stockholders.
| 91 |
| Table of Contents |
Nevada’s “combinations with interested stockholders” statutes, NRS 78.411 through 78.444, inclusive, prohibit specified types of business “combinations” between certain Nevada corporations and any person deemed to be an “interested stockholder” for two years after such person first becomes an “interested stockholder” unless the corporation’s board of directors approves the combination, or the transaction by which such person becomes an “interested stockholder”, in advance, or unless the combination is approved by the board of directors and sixty percent of the corporation’s voting power not beneficially owned by the interested stockholder, its affiliates and associates. Further, in the absence of prior approval certain restrictions may apply even after such two year period. However, these statutes do not apply to any combination of a corporation and an interested stockholder after the expiration of four years after the person first became an interested stockholder. For purposes of these statutes, an “interested stockholder” is any person who is (1) the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the outstanding voting shares of the corporation, or (2) an affiliate or associate of the corporation and at any time within the two previous years was the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the then outstanding shares of the corporation. The definition of the term “combination” is sufficiently broad to cover most significant transactions between a corporation and an “interested stockholder.” These statutes generally apply to Nevada corporations with 200 or more stockholders of record. However, a Nevada corporation may elect in its articles of incorporation not to be governed by these particular laws, but if such election is not made in the corporation’s original articles of incorporation, the amendment (1) must be approved by the affirmative vote of the holders of stock representing a majority of the outstanding voting power of the corporation not beneficially owned by interested stockholders or their affiliates and associates, and (2) is not effective until 18 months after the vote approving the amendment and does not apply to any combination with a person who first became an interested stockholder on or before the effective date of the amendment. We have made such an election in our original articles of incorporation.
Nevada’s “acquisition of controlling interest” statutes, NRS 78.378 through 78.379, inclusive, contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. These “control share” laws provide generally that any person that acquires a “controlling interest” in certain Nevada corporations may be denied voting rights, unless a majority of the disinterested stockholders of the corporation elects to restore such voting rights. Absent such provision in our bylaws, these laws would apply to us as of a particular date if we were to have 200 or more stockholders of record (at least 100 of whom have addresses in Nevada appearing on our stock ledger at all times during the 90 days immediately preceding that date) and do business in the State of Nevada directly or through an affiliated corporation, unless our articles of incorporation or bylaws in effect on the tenth day after the acquisition of a controlling interest provide otherwise. These laws provide that a person acquires a “controlling interest” whenever a person acquires shares of a subject corporation that, but for the application of these provisions of the NRS, would enable that person to exercise (1) one fifth or more, but less than one third, (2) one third or more, but less than a majority or (3) a majority or more, of all of the voting power of the corporation in the election of directors. Once an acquirer crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold and within the 90 days immediately preceding the date when the acquiring person acquired or offered to acquire a controlling interest become “control shares” to which the voting restrictions described above apply.
Nevada law also provides that directors may resist a change or potential change in control if the directors determine that the change is opposed to, or not in the best interests of, the corporation. The existence of the foregoing provisions and other potential anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of our Company, thereby reducing the likelihood that you could receive a premium for your common stock in an acquisition.
Anti-Takeover Effects of Our Articles of Incorporation and Bylaws
| 92 |
| Table of Contents |
The following provisions of our articles of incorporation and bylaws could have the effect of delaying or discouraging another party from acquiring control of us and could encourage persons seeking to acquire control of us to first negotiate with our Board :
|
| · | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
|
|
|
|
|
| · | the right of our Board to elect a director to fill a vacancy created by the expansion of the Board or the resignation, death or removal of a director, with our stockholders only allowed to fill such a vacancy if not filled by the Board; |
|
|
|
|
|
| · | the ability of our Board to alter our bylaws without obtaining shareholder approval; and |
|
|
|
|
|
| · | the requirement that a special meeting of stockholders may be called only by either (i) the Chairman; (ii) the President; (iii) Vice President, or (iv) at least two members of our Board, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors |
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company of Canada.
Stock Market Listing
Our common stock is currently quoted on OTCQX and trades on the CSE and under the symbols “LXRP” and “LXX,” respectively. We are in the process of applying to have our shares of common stock and warrants listed on Nasdaq under the symbols “LEXX” and “LEXXW”. There is no established public trading market for the warrants and pre-funded warrants, and we do not expect a market to develop for the pre-funded warrants. There is no assurance that a market will develop for the warrants. In addition, we do not intend to apply to list the pre-funded warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the prefunded warrants will be limited.
Indemnification of Directors and Officers
The NRS empower us to indemnify our directors and officers against expenses relating to certain actions, suits or proceedings as provided for therein. In order for such indemnification to be available, the applicable director or officer must not have acted in a manner that constituted a breach of his or her fiduciary duties and involved intentional misconduct, fraud or a knowing violation of law, or must have acted in good faith and reasonably believed that his or her conduct was in, or not opposed to, our best interests. In the event of a criminal action, the applicable director or officer must not have had reasonable cause to believe his or her conduct was unlawful.
Pursuant to our articles, we may indemnify each of our present and future directors, officers, employees or agents who becomes a party or is threatened to be made a party to any suit or proceeding, whether pending, completed or merely threatened, and whether said suit or proceeding is civil, criminal, administrative, investigative, or otherwise, except an action by or in the right of the Company, by reason of the fact that he is or was a director, officer, employee, or agent of the Company, or is or was serving at the request of the Company as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise, against expenses, including, but not limited to, attorneys' fees, judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit, proceeding or settlement, provided such person acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interest of the Company, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
| 93 |
| Table of Contents |
The expenses of directors, officers, employees or agents of the Company incurred in defending a civil or criminal action, suit, or proceeding may be paid by the Company as they are incurred and in advance of the final disposition of the action, suit, or proceeding, if and only if the director, officer, employee or agent undertakes to repay said expenses to the Company if it is ultimately determined by a court of competent jurisdiction, after exhaustion of all appeals therefrom, that he is not entitled to be indemnified by the corporation.
No indemnification shall be applied, and any advancement of expenses to or on behalf of any director, officer, employee or agent must be returned to the Company, if a final adjudication establishes that the person's acts or omissions involved a breach of any fiduciary duties, where applicable, intentional misconduct, fraud or a knowing violation of the law which was material to the cause of action.
The NRS further provides that a corporation may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out of his status as such, whether or not the corporation has the authority to indemnify him against such liability and expenses. We have secured a directors’ and officers’ liability insurance policy. We expect that we will continue to maintain such a policy.
Disclosure of Commission Position on Indemnification for Securities Act Liabilities
Insofar as indemnification for liabilities under the Securities Act may be permitted to officers, directors or persons controlling the Company pursuant to the foregoing provisions, the Company has been informed that is it is the opinion of the SEC that such indemnification is against public policy as expressed in such Securities Act and is, therefore, unenforceable.
RESTRICTIONS ON RESALE TO RESIDENTS OF CANADA
The Company is a “reporting issuer” (within the meaning of applicable Canadian securities laws) in each of the provinces of British Columbia and Ontario . However, as this offering of securities is being made solely outside of Canada, the Company is exempt from the requirement to prepare and file a prospectus with the securities regulatory authorities in each of the provinces of Canada to qualify the distribution of the securities. Accordingly, each purchaser of the securities acknowledges that the securities are subject to “hold period” resale restrictions under applicable Canadian securities laws such that such securities must not be traded or resold in or to a resident of Canada until four months and a day after the closing of the offering, and each purchaser of securities agrees and is deemed to agree to comply with such restrictions. Accordingly, this prospectus supplement serves as notice to each purchaser of securities of the transfer and resale restrictions applicable to the securities under Canadian securities laws described in the following legend:
“UNDER CANADIAN SECURITIES LAWS, UNLESS PERMITTED UNDER SECURITIES LEGISLATION, THE HOLDER OF THIS SECURITY MUST NOT TRADE THE SECURITY IN CANADA BEFORE THE DATE THAT IS 4 MONTHS AND A DAY AFTER THE ORIGINAL DISTRIBUTION DATE OF THE SECURITIES.”
The validity of the issuance of securities offered by this prospectus has been passed upon for us by Sichenzia Ross Ference LLP, New York, New York. Ellenoff Grossman & Schole LLP is acting as counsel to the underwriters.
| 94 |
| Table of Contents |
The audited consolidated financial statements of the Company and its subsidiaries, as of and for the years ended August 31, 2020 and 2019 included in this prospectus have been so included in reliance upon the report of Davidson & Company LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file reports, proxy statements and other information with the SEC. The SEC maintains an internet site that contains reports, proxy and information statements and other information regarding Lexaria Bioscience Corp. and other issuers that file electronically with the SEC. The address of the SEC internet site is www.sec.gov. In addition, we make available on or through our Internet site copies of these reports as soon as reasonably practicable after we electronically file or furnish them to the SEC. Our Internet site can be found at www.lexariabioscience.com
| 95 |
| Table of Contents |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
| Page |
| |
|
|
|
|
| |
| Consolidated Financial Statements as of August 31, 2020 and 2019 |
|
|
| |
|
|
|
|
| |
|
|
| F-2 |
| |
|
|
|
|
|
|
|
| F-3 |
| ||
|
|
|
|
|
|
|
| F-4 |
| ||
|
|
|
|
|
|
|
| F-5 |
| ||
|
|
|
|
|
|
|
| F-6 |
| ||
|
|
|
|
|
|
|
| F-7 - F-26 |
| ||
| F-1 |
| Table of Contents |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Directors of
Lexaria Bioscience Corp.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Lexaria Bioscience Corp. (the “Company”), as of August 31, 2020 and 2019, and the related consolidated statements of operations and comprehensive loss, cash flows and stockholders’ equity for the years ended August 31, 2020 and 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Lexaria Bioscience Corp. as of August 31, 2020 and 2019, and the results of its operations and its cash flows for the years ended August 31, 2020 and 2019 in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatements of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company’s auditor since 2016.
“DAVIDSON & COMPANY LLP”
Chartered Professional Accountants
Vancouver, Canada
October 14, 2020
| F-2 |
| Table of Contents |
| LEXARIA BIOSCIENCE CORP. | ||||||||
| (Expressed in U.S. Dollars) | ||||||||
|
|
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
| ASSETS |
|
|
|
|
|
| ||
| Current |
|
|
|
|
|
| ||
| Cash and cash equivalents |
| $ | 1,293,749 |
|
| $ | 1,285,147 |
|
| Marketable securities (Note 19) |
|
| 19,321 |
|
|
| 64,214 |
|
| Accounts receivable (Note 7) |
|
| 313,925 |
|
|
| 273,145 |
|
| Inventory (Note 8) |
|
| 116,871 |
|
|
| 127,396 |
|
| Prepaid expenses and deposit (Note 18) |
|
| 182,095 |
|
|
| 68,927 |
|
| Total Current Assets |
|
| 1,925,961 |
|
|
| 1,818,829 |
|
|
|
|
|
|
|
|
|
|
|
| Non-current assets, net |
|
|
|
|
|
|
|
|
| Lease right of use |
|
| 126,920 |
|
|
| - |
|
| Intellectual property (Note 9) |
|
| 292,000 |
|
|
| 265,127 |
|
| Property & equipment (Note 10) |
|
| 483,357 |
|
|
| 591,263 |
|
| Total Non-current Assets |
|
| 902,277 |
|
|
| 856,390 |
|
| TOTAL ASSETS |
| $ | 2,828,238 |
|
| $ | 2,675,219 |
|
|
|
|
|
|
|
|
|
|
|
| LIABILITIES |
|
|
|
|
|
|
|
|
| Current |
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities (Note 11) |
| $ | 86,920 |
|
| $ | 136,411 |
|
| Deferred revenue (Note 14) |
|
| 44,255 |
|
|
| - |
|
| Due to related party (Note 15) |
|
| 58,704 |
|
|
| 48,096 |
|
| Lease current (Note 17) |
|
| 36,038 |
|
|
| - |
|
| Total Current Liabilities |
|
| 225,917 |
|
|
| 184,507 |
|
|
|
|
|
|
|
|
|
|
|
| Long Term |
|
|
|
|
|
|
|
|
| Lease long term(Note 17) |
|
| 89,393 |
|
|
| - |
|
| Loan payable |
|
| 30,670 |
|
|
| - |
|
| Total Long Term Liabilities |
|
| 120,063 |
|
|
| - |
|
| TOTAL LIABILITIES |
|
| 345,980 |
|
|
| 184,507 |
|
|
|
|
|
|
|
|
|
|
|
| STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
| Share capital (Note 12) |
|
|
|
|
|
|
|
|
| Authorized: |
|
|
|
|
|
|
|
|
| 220,000,000 common voting shares with a par value of $0.001 per share |
|
|
|
|
|
|
|
|
| Issued and outstanding:90,044,312 common shares at August 31, 2020 and 78,787,134 common shares at August 31, 2019 |
|
| 90,044 |
|
|
| 78,787 |
|
| Additional paid-in capital (Note 12, 13) |
|
| 30,237,355 |
|
|
| 26,172,453 |
|
| Deficit |
|
| (27,802,198 | ) |
|
| (23,868,202 | ) |
| Equity attributable to shareholders of the Company |
|
| 2,525,201 |
|
|
| 2,383,038 |
|
| Non-Controlling Interest |
|
| (42,943 | ) |
|
| 107,674 |
|
| Total Stockholders' Equity |
|
| 2,482,258 |
|
|
| 2,490,712 |
|
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
| $ | 2,828,238 |
|
| $ | 2,675,219 |
|
The accompanying notes are an integral party of these consolidated financial statements.

| F-3 |
| Table of Contents |
| LEXARIA BIOSCIENCE CORP. | ||||||||
| CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS | ||||||||
| (Expressed in U.S. Dollars, except number of shares) | ||||||||
|
|
|
|
|
| ||||
|
|
| YEAR ENDED |
| |||||
|
|
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
| Revenue (Note 14) |
| $ | 384,543 |
|
| $ | 222,610 |
|
| Cost of goods sold |
|
| 99,378 |
|
|
| 22,893 |
|
| Gross profit |
|
| 285,165 |
|
|
| 199,717 |
|
|
|
|
|
|
|
|
|
|
|
| Expenses |
|
|
|
|
|
|
|
|
| Accounting and audit |
|
| 78,650 |
|
|
| 77,388 |
|
| Depreciation and amortization (Note 9, 10) |
|
| 112,750 |
|
|
| 60,550 |
|
| Advertising and promotions |
|
| 204,277 |
|
|
| 515,360 |
|
| Bad debt |
|
| 50,000 |
|
|
| 75,000 |
|
| Consulting (Notes 13, 15, 17) |
|
| 2,193,076 |
|
|
| 1,444,735 |
|
| Investor relations |
|
| 184,277 |
|
|
| 203,893 |
|
| Legal and professional |
|
| 371,844 |
|
|
| 670,863 |
|
| Office and miscellaneous |
|
| 292,880 |
|
|
| 297,209 |
|
| Research and development |
|
| 387,074 |
|
|
| 555,730 |
|
| Travel |
|
| 47,336 |
|
|
| 100,587 |
|
| Wages & salaries |
|
| 401,283 |
|
|
| 333,199 |
|
| Loss on disposal of marketable securities |
|
| 18,198 |
|
|
| - |
|
| Unrealized loss on marketable securities (Note 19) |
|
| 19,893 |
|
|
| 16,434 |
|
| Inventory writeoff (Note 8) |
|
| 8,240 |
|
|
| 7,182 |
|
|
|
|
| 4,369,778 |
|
|
| 4,358,130 |
|
|
|
|
|
|
|
|
|
|
|
| Net (loss) and comprehensive loss for the year |
| $ | (4,084,613 | ) |
| $ | (4,158,413 | ) |
| Net (loss) and comprehensive loss attributable to: |
|
|
|
|
|
|
|
|
| Common shareholders |
| $ | (3,933,996 | ) |
| $ | (4,099,420 | ) |
| Non-controlling interest |
| $ | (150,617 | ) |
| $ | (58,993 | ) |
|
|
|
|
|
|
|
|
|
|
| Basic and diluted (loss) per share |
| $ | (0.05 | ) |
| $ | (0.05 | ) |
|
|
|
|
|
|
|
|
|
|
| Weighted average number of common shares outstanding |
|
|
|
|
|
|
|
|
| - Basic and diluted |
|
| 83,201,271 |
|
|
| 77,792,263 |
|
The accompanying notes are an integral party of these consolidated financial statements.

| F-4 |
| Table of Contents |
| LEXARIA BIOSCIENCE CORP. | ||||||||
| (Expressed in U.S. Dollars) | ||||||||
|
|
|
|
| |||||
|
|
| YEAR ENDED |
| |||||
|
|
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
| Cash flows used in operating activities |
|
|
|
|
|
| ||
| Net loss and comprehensive loss |
| $ | (4,084,613 | ) |
| $ | (4,158,413 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
| Stock based compensation |
|
| 1,139,270 |
|
|
| 626,692 |
|
| Depreciation and amortization (Note 8, 9, 10) |
|
| 112,750 |
|
|
| 60,550 |
|
| Inventory write-off (Note 8) |
|
| 8,240 |
|
|
| 7,182 |
|
| Bad debt expense |
|
| 50,000 |
|
|
| 75,000 |
|
| Noncash right of use lease expense |
|
| (34,831 | ) |
|
| - |
|
| Realized loss on disposal of marketable securities (Note 19) |
|
| 18,198 |
|
|
| - |
|
| Unrealized loss on marketable securities (Note 19) |
|
| 19,893 |
|
|
| 16,434 |
|
| Common shares issued for services |
|
| 100,000 |
|
|
| 234,500 |
|
| Warrants issued for services |
|
| 168,833 |
|
|
| 52,817 |
|
| Change in working capital |
|
|
|
|
|
|
|
|
| Accounts receivable |
|
| (90,780 | ) |
|
| (138,644 | ) |
| Inventory |
|
| 4,213 |
|
|
| (47,345 | ) |
| Prepaid expenses and deposits |
|
| (113,168 | ) |
|
| 124,805 |
|
| Accounts payable and accrued liabilities |
|
| (49,491 | ) |
|
| 100,626 |
|
| Due to related parties |
|
| 10,608 |
|
|
| 40,241 |
|
| Operating lease liability |
|
| 33,342 |
|
|
| - |
|
| Deferred revenue |
|
| 44,255 |
|
|
| - |
|
| Net cash used in operating activities |
| $ | (2,663,281 | ) |
| $ | (3,005,555 | ) |
|
|
|
|
|
|
|
|
|
|
| Cash flows used in investing activities |
|
|
|
|
|
|
|
|
| Sale of marketable securities (Note 20) |
|
| 6,802 |
|
|
| - |
|
| Intellectual Property |
|
| (33,645 | ) |
|
| (122,982 | ) |
| Property & equipment |
|
| - |
|
|
| (646,183 | ) |
| Net cash used in investing activities |
| $ | (26,843 | ) |
| $ | (769,165 | ) |
|
|
|
|
|
|
|
|
|
|
| Cash flows from financing activities |
|
|
|
|
|
|
|
|
| Investment from NCI |
|
| - |
|
|
| 1,000,000 |
|
| Long term loan |
|
| 30,670 |
|
|
| - |
|
| Proceeds from issuance of equity |
|
| 2,668,056 |
|
|
| 2,332,683 |
|
| Net cash from financing activities |
| $ | 2,698,726 |
|
| $ | 3,332,683 |
|
|
|
|
|
|
|
|
|
|
|
| Decrease in cash and cash equivalents |
|
| 8,602 |
|
|
| (442,037 | ) |
| Cash and cash equivalents, beginning of year |
|
| 1,285,147 |
|
|
| 1,727,184 |
|
| Cash and cash equivalents, end of year |
| $ | 1,293,749 |
|
| $ | 1,285,147 |
|
|
|
|
|
|
|
|
|
|
|
| Supplemental information of cash flows: |
|
|
|
|
|
|
|
|
| Income taxes paid in cash |
| $ | (12,978 | ) |
| $ | 13,919 |
|
| Reclassification of NCI to additional paid in capital on acquisition |
| $ | - |
|
| $ | 833,333 |
|
The accompanying notes are an integral party of these consolidated financial statements.

| F-5 |
| Table of Contents |
| LEXARIA BIOSCIENCE CORP. CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (Expressed in U.S. Dollars) |
| |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||
|
|
| COMMON STOCK |
|
|
|
|
|
|
| |||||||||||||||||||
|
|
| SHARES |
|
| AMOUNT $ |
|
| ADDITIONAL PAID-IN CAPITAL $ |
|
| DEFICIT $ |
|
| NCI $ |
|
| AOCI $ |
|
| TOTAL STOCKHOLDERS’ EQUITY $ |
| |||||||
| Balance August 31, 2018 |
|
| 75,533,471 |
|
|
| 75,533 |
|
|
| 22,095,682 |
|
|
| (19,768,782 | ) |
|
| - |
|
|
| (14,247 | ) |
|
| 2,388,186 |
|
| Shares issued for services |
|
| 250,000 |
|
|
| 250 |
|
|
| 234,250 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 234,500 |
|
| Stock based compensation |
|
| - |
|
|
| - |
|
|
| 626,692 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 626,692 |
|
| Warrants issued for services |
|
| - |
|
|
| - |
|
|
| 52,817 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 52,817 |
|
| Exercise of stock options |
|
| 430,000 |
|
|
| 430 |
|
|
| 65,820 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 66,250 |
|
| Exercise of warrants |
|
| 1,626,513 |
|
|
| 1,627 |
|
|
| 794,496 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 796,123 |
|
| Private Placement |
|
| 947,150 |
|
|
| 947 |
|
|
| 1,469,363 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,470,310 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (4,099,420 | ) |
|
| - |
|
|
| - |
|
|
| (4,099,420 | ) |
| Non-controlling interest |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (58,993 | ) |
|
| - |
|
|
| (58,993 | ) |
| Other comprehensive income |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 14,247 |
|
|
| 14,247 |
|
| Subsidiary Investment |
|
| - |
|
|
| - |
|
|
| 833,333 |
|
|
| - |
|
|
| 166,667 |
|
|
| - |
|
|
| 1,000,000 |
|
| Balance August 31, 2019 |
|
| 78,787,134 |
|
|
| 78,787 |
|
|
| 26,172,453 |
|
|
| (23,868,202 | ) |
|
| 107,674 |
|
|
| - |
|
|
| 2,490,712 |
|
| Shares issued for services |
|
| 347,222 |
|
|
| 347 |
|
|
| 99,653 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 100,000 |
|
| Stock based compensation |
|
| - |
|
|
| - |
|
|
| 1,139,270 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,139,270 |
|
| Warrants issued for services |
|
| - |
|
|
| - |
|
|
| 168,833 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 168,833 |
|
| Exercise of stock options |
|
| 220,000 |
|
|
| 220 |
|
|
| 29,810 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 30,030 |
|
| Private placement |
|
| 10,689,956 |
|
|
| 10,690 |
|
|
| 2,627,336 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 2,638,026 |
|
| Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (3,933,996 | ) |
|
| - |
|
|
| - |
|
|
| (3,933,996 | ) |
| Non-controlling interest |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (150,617 | ) |
|
| - |
|
|
| (150,617 | ) |
| Balance August 31, 2020 |
|
| 90,044,312 |
|
|
| 90,044 |
|
|
| 30,237,355 |
|
|
| (27,802,198 | ) |
|
| (42,943 | ) |
|
| - |
|
|
| 2,482,258 |
|
The accompanying notes are an integral part of these consolidated financial statements.

| F-6 |
| Table of Contents |
| LEXARIA BIOSCIENCE CORP. NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS August 31, 2020 (Expressed in U.S. Dollars) |
| 1. | Organization, Business and Going Concern |
|
|
|
|
| Lexaria Bioscience Corp. (“Lexaria”, or the “Company”) was formed on December 9, 2004 under the laws of the State of Nevada. In March of 2014, the Company began its entry into the bioscience and alternative health and wellness business. In May 2016, the Company commenced out-licensing its patented DehydraTECH™ technology (“DehydraTECH”) for improved delivery of bioactive compounds that promotes healthy ingestion methods, lower overall dosing and higher effectiveness in active molecule delivery. The Company has its office in Kelowna, BC, Canada.
The Company’s consolidated financial statements included herein have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission and in accordance with accounting principles generally accepted in the United States (US GAAP) applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The recurring losses from operations and net capital deficiency raise substantial doubt about the Company’s ability to continue as a going concern.
The Company requires additional funds to maintain its operations and developments. Management’s plans in this regard are to raise equity and debt financing as required, but there is no certainty that such financing will be available or that it will be available at acceptable terms. The outcome of these matters cannot be predicted at this time.
In March 2020, the World Health Organization declared coronavirus COVID-19 a global pandemic. This contagious disease outbreak and any related adverse public health developments may adversely affect workforces, economies, and financial markets globally, potentially leading to an economic downturn. It is not possible for the Company to predict the duration or magnitude of the adverse results of the outbreak and its effects on the Company’s business or results of operations at this time. |
| 2. | Business Risk and Liquidity |
|
|
|
|
| The Company is subject to several categories of risk associated with its operating activities. Although we intend to develop our businesses in accordance with best ethical practices, we may suffer negative publicity if we, our partners, contractors, or customers are found to have engaged in any environmentally insensitive practices or other business practices that are viewed as unethical.
Our operations may require licenses and permits from various governmental authorities. We believe that we will be able to obtain all necessary licenses and permits under applicable laws and regulations for our operations and believe we will be able to comply in all material respects with the terms of such licenses and permits. However, such licenses and permits are subject to change in various circumstances. There can be no guarantee that we will be able to obtain or maintain all necessary licenses and permits and failing to obtain or retain required licenses could have a materially adverse effect on the Company. |

| F-7 |
| Table of Contents |
|
| Lexaria and its subsidiaries are not involved directly or indirectly in the cultivation, processing, distribution, or utilization of cannabis or cannabis derived components. Lexaria does have an ancillary involvement risk via out-licensing of its patented technology to licensees that choose to utilize DehydraTECH to manufacture products that contain locally or state approved but federally regulated and controlled contents. There can be no guarantee that changes in the regulatory framework and environment will not occur and such changes could have a materially adverse effect on the Company.
Lexaria and its subsidiaries are not involved directly or indirectly in the production or sale of any products containing nicotine. Products containing nicotine have historically been involved in litigation in the USA. Lexaria’s corporate licensee may introduce products containing nicotine that utilize DehydraTECH to the US consumer market, which could therefore introduce third-party risks to Lexaria.
Lexaria and its subsidiaries are not involved directly or indirectly in the production or sale of any pharmaceutical or anti-viral products. Licensees may enhance their product’s delivery using our Technology, which could therefore introduce third-party risks to Lexaria. |
| 3. | Significant Accounting Policies |
|
|
|
|
| a) Accounting Principles
These consolidated financial statements have been prepared in conformity with generally accepted accounting principles of the United States of America. All amounts, unless otherwise stated, are in United States dollars.
b) Revenue Recognition
Product Revenue
Revenue from the sale of products is recognized when persuasive evidence of an arrangement exists, delivery has occurred, the sales price is fixed or determinable, and collectability is reasonably assured, which typically occurs upon shipment. The Company reports its sales net of the amount of actual sales returns. Sales tax collected from customers is excluded from net sales.
Licensing Revenue from Intellectual Property
We recognize revenue for license fees at a point in time following the transfer of our intellectual property, namely our patented lipid nutrient infusion technology DehydraTECH for infusing Active Pharmaceutical Ingredients (“API”), to the licensee, which typically occurs on delivery of documentation.
Usage Fees from Intellectual Property
We recognize revenue for usage fees when usage of our DehydraTECH intellectual property occurs by licensees infusing an API into one or more of their product lines for sale.
c) Inventory and Cost of Sales
The Company’s inventory consists of finished goods, work in progress, and raw materials. In all classes, inventory is valued at the lower of cost or market. Cost is determined on a first-in, first-out basis.
Cost of sales includes all expenditures incurred in bringing the goods to the point of sale. Inventory costs and costs of sales include direct costs of the raw material, inbound freight charges, warehousing costs, handling costs (receiving and purchasing), utilities and overhead expenses. |

| F-8 |
| Table of Contents |
|
| d) Cash and Cash Equivalents
Cash equivalents comprise certain highly liquid instruments with a maturity of three months or less when purchased. As of August 31, 2020, and August 31, 2019, the Company held cash only.
e) Equipment
Equipment is stated at cost less accumulated depreciation and impairment, and depreciated using the straight-line method over their useful lives or by units of production.
f) Patents
Capitalized patent costs represent legal costs incurred to establish patents. When patents reach a mature stage, any associated legal costs are comprised mostly of maintenance fees and are expensed as incurred. Capitalized patent costs are amortized on a straight-line basis over the remaining life of the patent. The Company was granted its first patent on October 25, 2016, with a legal life of 20 years from the patent application’s filing date . Additional patent information is in Note 9.
g) Stock-Based Compensation
The Company accounts for its stock-based compensation awards in accordance with ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees, including grants of employee stock options, to be recognized as expenses in the statements of operations based on their grant date fair values. For stock options granted to employees and to members of the Boardfor their services on the Board, the Company estimates the grant date fair value of each option award using the Black-Scholes option-pricing model. The use of the Black-Scholes option-pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk-free interest rates and expected dividend yields of the common stock.
Stock-based payments issued to non-employees are recorded at their fair values and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period in accordance with the provisions of ASC 718 and ASC Topic 505, Equity. For equity instruments granted the Company recognizes stock-based compensation expense on vesting.
h) Loss Per Share
The Company applies the guidance in ASC 260 Earnings Per Share. Loss per share is computed using the weighted average number of shares outstanding during the year. Diluted loss per share is equivalent to basic loss per share because the potential exercise of the equity-based financial instruments was anti-dilutive. |

| F-9 |
| Table of Contents |
|
| i) Foreign Currency Translation
The Company’s operations are located in the United States of America and Canada, and it has offices in Canada. The Company maintains its accounting records in U.S. Dollars, as follows:
At the transaction date, each asset, liability, revenue and expense that was acquired or incurred in a foreign currency is translated into U.S. dollars by using the exchange rate in effect at that date. At the year end, monetary assets and liabilities are translated at the exchange rate in effect at that date. The resulting foreign exchange gains and losses are included in profit or loss.
j) Financial Instruments
ASC 820 Fair Value Measurements and Disclosures, requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1 - Quoted prices in active markets for identical assets or liabilities;
Level 2 - Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable; and
Level 3 - Unobservable inputs that are supported by little or no market activity, therefore requiring an entity to develop its own assumptions about the assumptions that market participants would use in pricing.
The Company’s financial instruments consist primarily of cash, marketable securities, accounts receivable, accounts payable and accrued liabilities, and due to related parties. The carrying amounts of cash, accounts and other receivable, accounts payable and accrued liabilities, and due to related parties approximate their fair values due to their short maturities or quoted market prices.
The Company is located in Canada, which results in exposure to market risks from changes in foreign currency rates. The foreign currency exchange risk is the financial risk to the Company’s operations that arise from fluctuations in foreign exchange rates and the degree of volatility of these rates. Currently, the Company does not use derivative instruments to reduce its exposure to foreign currency risk as the Company does not hold a significant position in foreign currencies, such as the Canadian dollar, and the impact of a change in a few basis points for USD/CAD is not expected to be material.
k) Income Taxes
The Company applies the guidance in ASC 740, Income Taxes, which requires the Company to recognize deferred tax liabilities and assets for the expected future tax consequences of events that have been recognized in the Company’s financial statements or tax returns using the liability method. Under this method, deferred tax liabilities and assets are determined based on the temporary differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect in the year in which the differences are expected to reverse. |

| F-10 |
| Table of Contents |
|
| l) Impairment of Long-Lived Assets
Long-lived assets, including equipment, and intangible assets, such as the Company’s patents, are assessed for potential impairment when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. An impairment loss is recognized when the carrying amount of the long-lived asset is not recoverable and exceeds its fair value. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. Any required impairment loss is measured as the amount by which the carrying amount of the long-lived asset exceeds its fair value and is recorded as a reduction in the carrying value of the related asset and a charge to the profit or loss. Intangible assets with indefinite lives are tested for impairment annually and in interim periods if certain events occur indicating that the carrying value of the intangible assets may be impaired.
m) Comprehensive Income
The Company applies ASC 220, Comprehensive Income, which establishes standards for reporting and presentation of comprehensive income, its components and accumulated balances. The Company discloses this information on its Statement of Stockholders’ Equity. Comprehensive income comprises equity changes except those transactions resulting from investments by owners and distributions to owners.
n) Credit Risk and Receivable Concentration
The Company places its cash with a high credit quality financial institution. As of August 31, 2020, the Company had approximately $1,293,749 in the bank (August 31, 2019: $1,285,147).
As at August 31, 2020 we had $143,500 (2019 – $106,000) in IP Territory license fees receivable (Note 7) consisting of amounts due from three licensees (2019 – three). These receivable amounts are based on contractual terms for payments that are payable within twelve months of signing the definitive agreements or routine IP usage fees. To date these licensees have performed all of their required obligations. The Company incurred $50,000 in bad debt in fiscal 2020 (2019 – $75,000).
As at August 31, 2020, the Company had $87,933 (2019 - $161,418) in sales tax receivable (Note 7). The Company considers its credit risk to be low for such receivables.
o) Commitments and Contingencies
In accordance with ASC 450-20, Accounting for Contingencies, the Company records accruals for such loss contingencies when it is probable that a liability has been incurred and the amount of loss can be reasonably estimated. In the event that estimates or assumptions prove to differ from actual results, adjustments are made in subsequent periods to reflect more current information. Historically, the Company has not experienced any material claims. |

| F-11 |
| Table of Contents |
|
| p) Research and Development
Research and development costs are expensed as incurred.
q) Leases
On September 1, 2019, we adopted ASC Topic 842, Leases (“ASC 842”) using the optional transition method and applied the standard only to leases that existed at that date. Under the optional transition method, we do not need to restate the comparative periods in transition and will continue to present financial information and disclosures for periods before September 1, 2019, in accordance with ASC Topic 840. We have elected the package of practical expedients allowed under ASC Topic 842, which permits us to account for our existing operating leases as operating leases under the new guidance, without reassessing our prior conclusions about lease identification, lease classification and initial direct cost. As a result of the adoption of the new lease accounting guidance, we recognized on September 1, 2019, operating lease right-of-use assets of $160,289 and operating lease liabilities of $158,773.
We determined the initial classification and measurement of our right-of-use assets and lease liabilities at the lease commencement date and thereafter if modified. The lease term includes any renewal options and termination options that we are reasonably certain to exercise. The present value of lease payments is determined by using the interest rate implicit in the lease, if that rate is readily determinable; otherwise, we use our incremental borrowing rate. The incremental borrowing rate is determined by using the rate of interest that we would pay to borrow on a collateralized basis an amount equal to the lease payments for a similar term and in a similar economic environment.
Rent expense for operating leases is recognized on a straight-line basis, unless the right-of-use asset has been impaired, over the reasonably certain lease term based on the total lease payments and is included in operating expenses in the consolidated statements of operations and comprehensive loss.
For operating leases that reflect impairment, we will recognize the amortization of the right-of-use asset on a straight-lined basis over the remaining lease term with rent expense still included in operating expenses in the consolidated statements of operations and comprehensive loss.
For all leases, rent payments that are based on a fixed index or rate at the lease commencement date are included in the measurement of lease assets and lease liabilities at the lease commencement date.
We have elected the practical expedient to not separate lease and non-lease components. Our non-lease components are primarily related to property taxes and maintenance, which vary based on future outcomes, and thus differences to original estimates are recognized in rent expense when incurred. |
| 4. | Basis of Consolidation |
|
|
|
|
| These consolidated financial statements include the financial statements of the Company and its wholly-owned subsidiaries; Lexaria CanPharm ULC, PoViva Corp., Lexaria Hemp Corp., Kelowna Management Services Corp. and Lexaria Pharmaceutical Corp., and our 83.333% subsidiary Lexaria Nicotine LLC (16.667% Altria Ventures Inc., an indirect wholly-owned subsidiary of Altria Group, Inc.). All significant intercompany balances and transactions have been eliminated. |

| F-12 |
| Table of Contents |
| 5. | Estimates and Judgments |
|
|
|
|
| The preparation of financial statements in conformity with U.S GAAP requires us to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting year. Some of the Company’s accounting policies require us to make subjective judgments, often as a result of the need to make estimates of matters that are inherently uncertain. These accounting policies involve critical accounting estimates because they are particularly dependent on estimates and assumptions made by management about matters that are highly uncertain at the time the accounting estimates are made. Although we have used our best estimates based on facts and circumstances available to us at the time, different estimates reasonably could have been used. Changes in the accounting estimates used by the Company are reasonably likely to occur from time to time, which may have a material effect on the presentation of financial condition and results of operations.
The Company reviews these estimates, judgments and assumptions periodically and reflect the effects of revisions in the period in which they are deemed to be necessary. We believe that these estimates are reasonable; however, actual results could differ from these estimates.
Significant accounting estimates and assumptions are used for, but not limited to: |
|
|
|
|
| a) The Valuation of Deferred Tax Assets
Judgement is required in determining whether deferred tax assets are recognized on the balance sheet. The recognition of deferred tax assets requires management to assess the likelihood that the Company will generate taxable income in future periods to utilize the deferred tax assets. Due to the Company’s history of losses, deferred tax assets have not been recognized by Lexaria.
b) Value of Stock Options and Warrants
The Company provides compensation benefits to its employees, directors, officers, and consultants, through a stock option plan. The fair value of each option award is estimated on the date of grant using the Black-Scholes option pricing model. Expected volatility assumptions used in the model are based on the historical volatility of the Company’s share price. The Company uses historical data to estimate the period of option exercises for use in the valuation model. The risk-free interest rate for the expected term of the option is based on the yields of government bonds. Changes in these assumptions, especially the share price volatility and the expected life determination could have a material impact on the Company’s profit and loss for the years presented. All estimates used in the model are based on historical data which may not be representative of future results. |

| F-13 |
| Table of Contents |
| 6. | Recent Accounting Guidance |
|
|
|
|
| In February 2016 FASB issued ASU No. 201602, Leases (Topic 842) which supersedes FASB ASC Topic 840, Leases (Topic 840) and provides principles for the recognition, measurement, presentation, and disclosure of leases for both lessees and the lessors. The new standard requires the lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. The classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease, respectively. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than twelve months regardless of classification. Leases with a term of twelve months or less will be accounted for similar to existing guidance for operating leases. In November 2019 FASB issued ASU No 201910 revised the effective date based on updated criteria with the effective date for fiscal years beginning after December 15, 2020. In June 2020 FASB issued ASU No 202005 further delaying the effective date for fiscal years beginning after December 15, 2021 due to the COVID-19 pandemic. The Company has adopted this standard as of August 31, 2020 (Note 17).
In June 2016, the FASB issued a new standard to replace the incurred loss impairment methodology in current U.S. GAAP with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. For trade and other receivables, loans and other financial instruments, the Company will be required to use a forward-looking expected loss model rather than the incurred loss model for recognizing credit losses which reflects losses that are probable. Credit losses relating to available for sale debt securities will also be recorded through an allowance for credit losses rather than as a reduction in the amortized cost basis of the securities. In November 2019 FASB issued ASU No 201910 revised the effective date based on updated criteria with the effective date for fiscal years beginning after December 15, 2022. Application of the amendments is through a cumulative effect adjustment to deficit as of the effective date. The Company is currently assessing the impact of the standard on its consolidated financial statements.
In February 2018, the FASB issued ASU No. 201802, Income Statement–Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income, which allows a reclassification from accumulated other comprehensive income to retained earnings for stranded tax effects resulting from the Tax Cuts and Jobs Act enacted by the U.S. federal government on December 22, 2017 (the “2017 Tax Act”). Consequently, the amendments eliminate the stranded tax effects resulting from the 2017 Tax Act and will improve the usefulness of information reported to financial statement users. The amendments in this ASU are effective for all entities for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted, including adoption in any interim period, (1) for public business entities for reporting periods for which financial statements have not yet been issued and (2) for all other entities for reporting periods for which financial statements have not yet been made available for issuance. The Company adopted the ASU on September 1, 2019 for a $NIL effect.
In June 2018, the FASB issued ASU No. 201807, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share Based Payment Accounting. This is a simplification that involves several aspects of accounting for nonemployee share-based payments resulting from expanding the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. The Company adopted the ASU on September 1, 2019 for a $NIL effect. |

| F-14 |
| Table of Contents |
| 7. | Accounts and Other Receivables |
|
|
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
|
|
| $ |
|
| $ |
| ||
| Trade and deposits receivable |
|
| 82,492 |
|
|
| 5,727 |
|
| Territory license fee receivable |
|
| 143,500 |
|
|
| 106,000 |
|
| Sales tax receivable |
|
| 87,933 |
|
|
| 161,418 |
|
|
|
|
| 313,925 |
|
|
| 273,145 |
|
| 8. | Inventory |
|
|
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
|
|
| $ |
|
| $ |
| ||
| Raw materials |
|
| 51,404 |
|
|
| 45,068 |
|
| Work in progress |
|
| 15,705 |
|
|
| - |
|
| Finished goods |
|
| 49,762 |
|
|
| 82,328 |
|
|
|
|
| 116,871 |
|
|
| 127,396 |
|
|
| During the year ended August 31, 2020, the Company wrote down $8,240 (2019 - $7,182) of inventory to reflect its net realisable value. |
|
|
|
| 9. | Intellectual Property |
|
|
|
|
| The following is a list of US capitalized patents held by the Company |
| Issued Patent # | Patent Issuance Date | Patent Family |
| US 9,474,725 B1 | 10/25/2016 | Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof
|
| US 9,839,612 B2 | 12/12/2017 | |
| US 9,972,680 B2 | 05/15/2018 | |
| US 9,974,739 B2 | 05/22/2018 | |
| US 10,084,044 B2 | 09/25/2018 | |
| US 10,103,225 B2 | 10/16/2018 | |
| US 10,381,440 | 08/13/2019 | |
| US 10,374,036 | 08/06/2019 | |
| US 10,756,180 | 08/25/2020 |

| F-15 |
| Table of Contents |
|
| The Company also holds non-capitalized patents outside the US. A continuity schedule for patents is presented below: |
|
|
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
|
|
| $ |
|
| $ |
| ||
| Balance – Beginning |
|
| 265,127 |
|
|
| 146,538 |
|
| Addition |
|
| 33,645 |
|
|
| 122,982 |
|
| Amortization* |
|
| (6,772 | ) |
|
| (4,393 | ) |
| Balance – Ending |
|
| 292,000 |
|
|
| 265,127 |
|
| *The patents are amortized over their legal life of 20 years from the patent application ’s filing date. |
|
|
|
|
|
|
|
|
| 10. | Property & Equipment |
| Year Ended August 31, 2020 |
| Cost |
|
| Amortization |
|
| Accumulated Amortization |
|
| Net Balance August 31, 2020 |
| ||||
|
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
| Leasehold improvements |
|
| 259,981 |
|
|
| (53,268 | ) |
|
| (86,610 | ) |
|
| 173,371 |
|
| Computers |
|
| 63,964 |
|
|
| (19,681 | ) |
|
| (31,869 | ) |
|
| 32,095 |
|
| Furniture fixtures equipment |
|
| 34,220 |
|
|
| (7,036 | ) |
|
| (13,097 | ) |
|
| 21,123 |
|
| Lab equipment |
|
| 291,235 |
|
|
| (27,921 | ) |
|
| (34,467 | ) |
|
| 256,768 |
|
|
|
|
| 649,400 |
|
|
| (107,906 | ) |
|
| (166,043 | ) |
|
| 483,357 |
|
| Year Ended August 31, 2019 |
| Cost |
|
| Amortization |
|
| Accumulated Amortization |
|
| Net Balance August 31, 2019 |
| ||||
|
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
| Leasehold improvements |
|
| 259,981 |
|
|
| (33,342 | ) |
|
| (33,342 | ) |
|
| 226,639 |
|
| Computers |
|
| 63,964 |
|
|
| (12,187 | ) |
|
| (12,187 | ) |
|
| 51,777 |
|
| Furniture fixtures equipment |
|
| 34,220 |
|
|
| (4,205 | ) |
|
| (6,062 | ) |
|
| 28,158 |
|
| Lab equipment |
|
| 291,235 |
|
|
| (6,546 | ) |
|
| (6,546 | ) |
|
| 284,689 |
|
|
|
|
| 649,400 |
|
|
| (56,281 | ) |
|
| (58,137 | ) |
|
| 591,263 |
|
|
| During the period $1,928 of amortization was included in the cost of inventory. |

| F-16 |
| Table of Contents |
| 11. | Accounts Payable and Accrued Liabilities |
|
|
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
|
|
| $ |
|
| $ |
| ||
| Accounts Payable |
|
|
|
|
|
| ||
| Trades payable |
|
| 45,080 |
|
|
| 31,463 |
|
| Sales tax payable |
|
| - |
|
|
| 63,616 |
|
| Accrued Liabilities |
|
|
|
|
|
|
|
|
| Corporate tax payable |
|
| 3,834 |
|
|
| - |
|
| Trades payable |
|
| 38,006 |
|
|
| 41,332 |
|
| Total |
|
| 86,920 |
|
|
| 136,411 |
|
| 12. | Common Shares and Warrants |
|
|
|
|
| Fiscal 2020 Activity
During the year ended August 31, 2020, the Company closed, pursuant to two tranches, a non-brokered private placement for an aggregate total of 1,823,745 units priced at $0.45 each. Each unit consists of one common share and one share purchase warrant. Each warrant shall entitle the holder to acquire one common share of the Company for a period of two years at a price of $0.80 per Share until the first anniversary of issuance, and thereafter at a price of $1.20 until the second anniversary of issuance. The Company paid $3,938 and issued 8,750 broker warrants. The broker warrants have a term of 24 months and are each exercisable into one common share of the Company at a price of $0.80 per share until the first anniversary of issuance, and thereafter at a price of $1.20 until the second anniversary of issuance. The fair value of these broker warrants was determined to be $1,850, which were recorded as a share issuance cost within additional paid in capital for a net effect of $Nil.
The Company also issued an aggregate of 8,866,211 units at $0.23, issued in two tranches for gross proceeds of $2,039,229. Each unit consists of one common share and one full warrant. The warrants are exercisable on issuance at an exercise price of $0.35 with 8,028,254 expiring May 6, 2025 and 837,957 on May 11, 2025. Pursuant to the agent agreement $151,623 and 649,123 broker warrants with an exercise price of $0.35 expiring May 6, 2025, were paid. The broker warrants were valued at $128,329 and were recorded as a share issue cost within additional paid in capital for a net effect of $Nil. A total of $65,600 in legal fees were also paid.
The company granted a total of 500,000 warrants pursuant to an agreement with a consultant valued at $98,081 that were recorded as an expense within consulting.
The Company recognized $168,833 in consulting expense for warrants granted to consultants as per vesting requirements. |

| F-17 |
| Table of Contents |
|
| A summary of share issuance relating to exercises and private placements is presented below: |
| Type of Issuance |
| Number of Shares |
|
| Total Value $ |
| ||
| Option exercise |
|
| 220,000 |
|
|
| 30,030 |
|
| Private placement (1) |
|
| 10,689,956 |
|
|
| 2,859,914 |
|
| Per agreements(2) |
|
| 347,222 |
|
|
| 100,000 |
|
|
|
|
| 11,257,178 |
|
|
| 2,989,944 |
|
| (1) Total fees of $221,889 were paid for total net receipt of $2,638,025. |
| |||||||
| (2) The Company awarded the restricted common shares as required by consulting contracts. | ||||||||
|
| Fiscal 2019 Activity
During the year ended August 31, 2019 the Company closed a non-brokered private placement for 947,150 Units priced at $1.60 each. Each unit consists of one common share and one share purchase warrant. Each warrant shall entitle the holder to acquire one common share at a price of $2.25 per share for a period of 24 months. The Company also issued 28,175 broker warrants. The broker warrants have a term of 24 months and are each exercisable into one common share of the Company at a price of $2.25. The fair value of these broker warrants was determined to be $16,095, which were recorded as a share issue cost within additional paid in capital for a net effect of $Nil. The Company granted an additional 107,737 broker warrants with a value of $6,484 that were recorded as a share issue cost within additional paid in capital for a net effect of $Nil.
The Company granted a total of 100,000 warrants pursuant to an agreement with a vendor valued at $52,817 that were recorded as an expense within investor relation expense.
During the year ended August 31, 2019 the Company recognized $51,448 in consulting expense for warrants previously granted to a consultant upon vesting.
A summary of share issuance is presented relating to option and warrant exercises, agreement requirements and debt settlement is presented below: |
| Type of Issuance |
| Number of Shares |
|
| Total Value |
| ||
| Warrant exercise(1) |
|
| 1,626,513 |
|
|
| 796,122 |
|
| Option exercise |
|
| 430,000 |
|
|
| 66,250 |
|
| Private placement |
|
| 947,150 |
|
|
| 1,515,440 |
|
| Per agreements(2) |
|
| 250,000 |
|
|
| 234,500 |
|
|
|
|
| 3,253,663 |
|
| $ | 2,612,312 |
|
| (1) Includes 384,212 broker warrants exercised for gross proceeds of $191,742 |
|
|
|
|
|
|
|
|
| (2) The Company awarded the restricted common shares as required by consulting contracts. |
|
|
|
|
|
|
|
|

| F-18 |
| Table of Contents |
|
| A continuity schedule for warrants is presented below: |
|
|
| Number of Warrants |
|
| Weighted Average Exercise Price $ |
| ||
| Balance August 31, 2018 |
|
| 3,286,274 |
|
|
| 0.72 |
|
| Cancelled/Expired |
|
| (17,498 | ) |
|
| 0.59 |
|
| Exercised |
|
| (1,626,513 | ) |
|
| 0.49 |
|
| Issued |
|
| 1,183,062 |
|
|
| 1.99 |
|
| Balance August 31, 2019 |
|
| 2,825,325 |
|
|
| 1.38 |
|
| Cancelled/Expired |
|
| (750,000 | ) |
|
| 1.50 |
|
| Issued |
|
| 12,072,829 |
|
|
| 0.42 |
|
| Balance August 31, 2020 |
|
| 14,148,154 |
|
|
| 0.56 |
|
|
| The fair value of share purchase warrants granted as broker warrants, compensation units, and compensatory warrants, was estimated as of the date of the grant by using the Black-Scholes option pricing model with the following assumptions: |
|
|
| August 31 2020 |
|
| August 31 2019 |
| ||
| Expected volatility |
| 91%-94 | % |
| 1% – 117 | % | ||
| Risk-free interest rate |
| 0.36%-2.87 | % |
| 2.31% – 2.87 | % | ||
| Expected life |
| 2 – 5 years |
|
| 1 day – 2 years |
| ||
| Dividend yield |
|
| 0 | % |
|
| 0.00 | % |
| Estimated fair value per warrant |
| 0.28 – 0.54 |
|
| $Nil – $0.57 |
| ||
|
| A summary of warrants outstanding as of August 31, 2020 is presented below: |
| # of Warrants | Weighted Average Remaining Contractual Life | Weighted Average Exercise Price $ |
| 975,325 | 0.17 years | 2.25 |
| 100,000 | 0.72 years | 0.96 |
| 250,000 | 0.73 years | 1.55 |
| 750,000 | 1.11 years | 0.14 |
| 225,000 | 2.18 years | 0.80 |
| 1,562,995 | 1.20 years | 0.80 |
| 269,500 | 1.24 years | 0.80 |
| 500,000 | 4.54 years | 0.30 |
| 8,028,254 | 4.68 years | 0.35 |
| 1,487,080 | 4.70 years | 0.35 |
| 14,148,154 | 3.59 years | 0.56 |

| F-19 |
| Table of Contents |
| 13. | Stock Options |
|
|
|
|
| The Company has established its 2014 Stock Option Plan whereby the Board may, from time to time, grant up to 2,107,500 stock options to directors, officers, employees, and consultants, and the 2019 Equity Incentive Plan whereby the Board may, from time to time, grant up to 7,838,713 stock options to directors, officers, employees, and consultants. Stock options granted must be exercised no later than five years from the date of grant or such lesser period as determined by the Board . The exercise price of an option is equal to or greater than the closing market price of the Company’s common shares on the day preceding the date of grant. The vesting terms of each grant are set by the Board .
During the year ending August 31, 2020 the formerly established 2007 Equity Incentive Plan and the 2010 Stock Option Plan were cancelled. Outstanding options were cancelled and reissued under the 2019 Equity Incentive Plan.
Fiscal 2020 Activity
The Company granted stock options in the year ending August 31, 2020: |
| Quantity |
| Exercise Price $ | Life (Years) |
| 1,000,000 |
| 0.55 | 5 |
| 60,000 |
| 0.43 | 5 |
| 550,000 |
| 0.47 | 5 |
| 2,538,000 |
| 0.32 | 5 |
| 700,000 | 0. | 0.34 | 5 |
| 4,848,000 | (1) | 0.39 | 5 |
|
| (1) 3,962,000 have vested as at August 31, 2020, and 886,000 remain subject to vesting provisions. |
|
| Fiscal 2019 Activity
The Company granted stock options in the year ending August 31, 2019: |
| Quantity | Exercise Price $ | Life (Years) |
| 390,000(1) | 1.27 | 5 |
| 240,000(1) | 1.06 | 5 |
| 30,000(1) | 1.16 | 5 |
| 350,000 | 0.99 | 5 |
| 440,000(1) | 0.99 | 5 |
| 48,000(1) | 0.96 | 5 |
| 100,000 | 0.81 | 5 |
| 450,000(1) | 0.81 | 5 |
| 2,048,000 | 1.00 |
|
|
| (1) Options granted vest over a period of three years |

| F-20 |
| Table of Contents |
|
| A continuity schedule for stock options is presented below: |
|
|
| Options |
|
| Weighted Average Exercise Price $ |
|
| Weighted Average Remaining Contractual Term (Years) |
|
| Aggregate Intrinsic Value $ |
| ||||
| Balance August 31, 2018 |
|
| 4,800,000 |
|
|
| 0.71 |
|
|
|
|
|
|
| ||
| Expired/Cancelled |
|
| (1,415,000 | ) |
|
| 0.66 |
|
|
|
|
|
|
| ||
| Exercised |
|
| (430,000 | ) |
|
| 0.15 |
|
|
|
|
|
|
| ||
| Granted |
|
| 2,048,000 |
|
|
| 1.00 |
|
|
|
|
|
|
| ||
| Balance August 31, 2019 |
|
| 5,003,000 |
|
|
| 0.71 |
|
|
|
|
|
|
| ||
| Expired/Cancelled |
|
| (4,483,000 | ) |
|
| 0.98 |
|
|
|
|
|
|
| ||
| Exercised |
|
| (220,000 | ) |
|
| 0.14 |
|
|
|
|
|
|
| ||
| Granted |
|
| 4,848,000 |
|
|
| 0.39 |
|
|
|
|
|
|
| ||
| Balance August 31, 2020 (Outstanding) |
|
| 5,148,000 |
|
|
| 0.37 |
|
|
| 4.30 |
|
|
| 140,634 |
|
| Balance August 31, 2020 (Exercisable) |
|
| 4,262,000 |
|
|
| 0.34 |
|
|
| 4.29 |
|
|
| 136,853 |
|
|
| The fair value of options granted was estimated as of the date of the grant by using the Black-Scholes option pricing model with the following assumptions: |
|
|
| August 31 2020 |
|
| August 31 2019 |
| ||
| Expected volatility |
| 95% – 96 | % |
| 100% – 144 | % | ||
| Risk-free interest rate |
| 0.35% – 1.66 | % |
| 1.42% – 2.89 | % | ||
| Expected life |
| 5 years |
|
| 5 years |
| ||
| Dividend yield |
|
| 0 | % |
|
| 0 | % |
| Estimated fair value per option |
| $0.31-$0.54 |
|
| $0.60 - $1.07 |
| ||
| 14. | Revenues |
| August 31 2020 $ |
|
| August 31 2019 $ |
| ||||
| Product sales |
|
| 150,993 |
|
|
| 24,282 |
|
| Licensing revenue (Note 11) |
|
| 232,909 |
|
|
| 198,000 |
|
| Freight revenue |
|
| 641 |
|
|
| 328 |
|
|
|
|
| 384,543 |
|
|
| 222,610 |
|
|
| The Company recognized $232,909 of licensing revenue (2019 $198,000) and $150,993 of product revenues (2019 $24,282). Licensing revenue was significantly concentrated on one licensee and $121,906 of product revenues related to sales of our intermediate product for use by five customers in their products.
The licensing fees consist of IP licensing fees for transfer of the DehydraTECH technology with the signing of definitive agreements and usage fees. The licensing fees include payments due upon transfer of the technology and installment payments that are receivable within 12 months (Note 7). |

| F-21 |
| Table of Contents |
|
| As of August 31, 2020, we have $44,255 in deferred revenue from customers for production of intermediate products that are expected to be produced during our next fiscal quarter. |
|
|
|
| 15. | Related Party Transactions |
| Management, consulting and accounting services |
| Cash $ |
|
| % |
|
| Non-Cash(2) $ |
|
| % |
|
| Aug 31 2020 Total $ |
|
| Cash $ |
|
| % |
|
| Non-Cash (2) $ |
|
| % |
|
| Aug 31 2019 Total $ |
| ||||||||||
| C.A.B Financial Services(1) |
|
| 300,802 |
|
|
| 66 |
|
|
| 153,065 |
|
|
| 34 |
|
|
| 453,867 |
|
|
| 223,280 |
|
|
| 100 |
|
|
| - |
|
|
| - |
|
|
| 223,280 |
|
| M&E Services Ltd.(1) |
|
| 121,664 |
|
|
| 46 |
|
|
| 143,886 |
|
|
| 54 |
|
|
| 265,550 |
|
|
| 112,377 |
|
|
| 100 |
|
|
| - |
|
|
| - |
|
|
| 112,377 |
|
| Docherty Management Limited(1) |
|
| 242,521 |
|
|
| 47 |
|
|
| 275,614 |
|
|
| 53 |
|
|
| 518,135 |
|
|
| 195,740 |
|
|
| 100 |
|
|
| - |
|
|
| - |
|
|
| 195,740 |
|
| Company controlled by a director |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 14,932 |
|
|
| 12 |
|
|
| 112,718 |
|
|
| 88 |
|
|
| 127,650 |
|
| Directors |
|
| 67,146 |
|
|
| 43 |
|
|
| 88,544 |
|
|
| 57 |
|
|
| 155,690 |
|
|
| 16,138 |
|
|
| 9 |
|
|
| 172,330 |
|
|
| 91 |
|
|
| 188,468 |
|
|
|
|
| 732,133 |
|
|
|
|
|
|
| 661,109 |
|
|
|
|
|
|
| 1,393,242 |
|
|
| 562,467 |
|
|
|
|
|
|
| 285,048 |
|
|
|
|
|
|
| 847,515 |
|
|
| (1) C.A.B. Financial Services is owned by the CEO of the Company, M&E Services Ltd. is owned by the CFO of the Company, and Docherty Management Limited is owned by the President of the Company. (2) Stock Based Compensation (SBC) and Share Awards are included in the total value of the grants and awards included in expenses. In the year ended August 31, 2020 the Company granted $572,565 of option awards to officers and $88,544 awards to Directors included in Consulting expense replacing cancelled options (Note 13). |
|
| Due to related parties:
As at August 31, 2020, $58,704 (August 31, 2019 - $48,096) was payable to related parties included in due to related parties.
The related party transactions are recorded at the exchange amount established and agreed to between the related parties. |
|
|
|
| 16. | Segment Information |
|
|
|
|
| The Company’s operations involve the development and usage, including licensing, of DehydraTECH. Lexaria is centrally managed and its chief operating decision makers, being the President and the CEO, use the consolidated and other financial information supplemented by revenue information by category of alternative health consumer products and technology licensing to make operational decisions and to assess the performance of the Company. The Company has identified two reportable segments: Intellectual Property Licensing and Consumer Products. Licensing revenues are significantly concentrated on three licensees. |
|
|
| IP Licensing |
|
| Consumer Products |
|
| Corporate |
|
| Consolidated Total |
| ||||
|
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
| External revenue |
|
| 232,909 |
|
|
| 151,634 |
|
|
| - |
|
|
| 384,543 |
|
| Cost of goods sold |
|
| - |
|
|
| (99,378 | ) |
|
| - |
|
|
| (99,378 | ) |
| Operating expenses |
|
| (1,601,595 | ) |
|
| (1,043,956 | ) |
|
| (1,724,227 | ) |
|
| (4,369,778 | ) |
| Segment loss |
|
| (1,368,686 | ) |
|
| (991,700 | ) |
|
| (1,724,227 | ) |
|
| (4,084,613 | ) |
| Total assets |
|
| 692,268 |
|
|
| 116,871 |
|
|
| 2,019,099 |
|
|
| 2,828,238 |
|

| F-22 |
| Table of Contents |
| Capital Asset by Region |
| Cost |
|
| Net Balance |
|
| Cost |
|
| Net Balance Canada |
|
| Total Net Balance |
| |||||
| Year Ended August 31, 2020 |
| $ |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| |||||
| Leasehold Improvements |
|
| - |
|
|
| - |
|
|
| 259,981 |
|
|
| 173,371 |
|
|
| 173,371 |
|
| Computers |
|
| - |
|
|
| - |
|
|
| 63,964 |
|
|
| 32,095 |
|
|
| 32,095 |
|
| Furniture Fixtures Equipment |
|
| 3,094 |
|
|
| - |
|
|
| 31,126 |
|
|
| 21,123 |
|
|
| 21,123 |
|
| Lab Equipment |
|
| 98,050 |
|
|
| 85,264 |
|
|
| 193,185 |
|
|
| 171,505 |
|
|
| 256,769 |
|
|
|
|
| 101,144 |
|
|
| 85,264 |
|
|
| 548,256 |
|
|
| 398,094 |
|
|
| 483,358 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Capital Asset by Region |
| Cost |
|
| Net Balance |
|
| Cost |
|
| Net Balance Canada |
|
| Total Net Balance |
| |||||
| Year Ended August 31, 2019 |
| $ |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| |||||
| Leasehold Improvements |
|
| - |
|
|
| - |
|
|
| 259,981 |
|
|
| 226,638 |
|
|
| 226,638 |
|
| Computers |
|
| - |
|
|
| - |
|
|
| 63,964 |
|
|
| 51,776 |
|
|
| 51,776 |
|
| Furniture Fixtures Equipment |
|
| 3,094 |
|
|
| 619 |
|
|
| 31,126 |
|
|
| 27,540 |
|
|
| 28,159 |
|
| Lab Equipment |
|
| 98,050 |
|
|
| 85,420 |
|
|
| 193,185 |
|
|
| 199,269 |
|
|
| 284,689 |
|
|
|
|
| 101,144 |
|
|
| 86,039 |
|
|
| 548,256 |
|
|
| 505,223 |
|
|
| 591,262 |
|
| 17. | Commitments, Significant Contracts and Contingencies |
|
|
|
|
| Management and Service Agreements
As at August 31, 2020, the Company is party to the following contractual commitments: |
| Party | Monthly Commitment | Expiry Date | |
| C.A.B Financial Services |
| CAD $29,706 | January 1, 2022 |
| Docherty Management Ltd. |
| CAD $25,609 | January 1, 2022 |
| M&E Services Ltd. |
| CAD $13,997 | June 1, 2021 |
| Corporate Development |
| CAD $1,500 | Month to Month |
| Office Management |
| CAD $10,800 | August 15, 2022 |
| Research & Development |
| CAD $3,854 | Month to Month |
| Office operating lease(1) |
| CAD $4,823 | November 15, 2023 |
|
| Right of Use Assets - Operating Lease | |
|
| (1) Corporate office and R&D lab space leased in Kelowna, British Columbia, Canada until November 15, 2023 with an option to extend an additional five years. In addition to minimum lease payments, the lease requires us to pay property taxes and operating costs which are subject to annual adjustments. | |

| F-23 |
| Table of Contents |
| Right of use assets - operating leases: |
| $ |
| |
| September 1, 2019 |
|
| 160,289 |
|
| Amortization |
|
| (33,369 | ) |
| Total lease assets |
|
| 126,920 |
|
| Liabilities: |
|
|
|
|
| September 1, 2019 |
|
| 158,773 |
|
| Lease payments |
|
| (43,764 | ) |
| Interest accretion |
|
| 10,423 |
|
| Total lease liabilities |
|
| 125,431 |
|
|
|
|
|
|
|
| Operating lease cost as at August 31, 2020 |
| $ | 126,920 |
|
| Operating cash flows for lease |
| $ | 43,764 |
|
| Remaining lease term |
| 3.1 Years |
| |
| Discount rate |
|
| 7.25 | % |
|
| Pursuant to the terms of the Company’s lease agreements in effect at August 31, 2020, the following table summarizes the Company’s maturities of operating lease liabilities as of August 31, 2020: |
| 2021 |
|
| 43,950 |
|
| 2022 |
|
| 44,599 |
|
| 2023 |
|
| 44,815 |
|
| 2024 |
|
| 7,469 |
|
| Thereafter |
|
| - |
|
| Total lease payments |
|
| 140,832 |
|
| Less: imputed interest |
|
| (15,401 | ) |
| Present value of operating lease liabilities |
|
| 125,431 |
|
| Less: current obligations under leases |
|
| (36,038 | ) |
| Total |
|
| 89,393 |
|

| F-24 |
| Table of Contents |
| 18. | Prepaid Expenses |
|
|
|
|
| Prepaid expenses consist of the following as at August 31, 2020 and August 31, 2019: |
|
|
| August 31 |
|
| August 31 |
| ||
|
|
| 2020 |
|
| 2019 |
| ||
|
|
| $ |
|
| $ |
| ||
| Advertising & conferences |
|
| 21,878 |
|
|
| 39,143 |
|
| Legal fees |
|
| 47,498 |
|
|
| - |
|
| Licence, filing fees, dues |
|
| 8,541 |
|
|
| - |
|
| Office & insurance |
|
| 78,792 |
|
|
| 29,784 |
|
| Research & development |
|
| 25,386 |
|
|
| - |
|
|
|
|
| 182,095 |
|
|
| 68,927 |
|
| 19. | Marketable Securities |
|
|
|
|
| The components of Marketable Securities were as follows: |
|
|
| Cost Basis $ |
|
| Unrealized Gains $ |
|
| Unrealized Losses $ |
|
| Total $ |
| ||||
| August 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
| Common Stock |
|
| 81,250 |
|
|
| 9,335 |
|
|
| (12,124 | ) |
|
|
| |
| Total |
|
| 81,250 |
|
|
| 9,335 |
|
|
| (26,973 | ) |
|
| 63,612 |
|
| August 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common Stock |
|
| 56,250 |
|
|
| 9,997 |
|
|
| (38,584 | ) |
|
|
|
|
| Total |
|
| 56,250 |
|
|
| 9,997 |
|
|
| (46,926 | ) |
|
| 19,321 |
|
|
| We realized an $18,198 loss and received $6,802 in net proceeds on the sale of marketable securities.
Unrealized losses from common stock are due to market price movements. Management does not believe any remaining unrealized losses represent other-than-temporary impairments based on our evaluation of available evidence. |

| F-25 |
| Table of Contents |
| 20. | Income Tax |
|
|
|
|
| The following table reconciles the income tax benefit at the U.S. Federal statutory rate to income tax benefit at the Company’s effective tax rates as at August 31, 2020 and 2019: |
|
|
| August 31 2020 $ |
|
| August 31 2019 $ |
| ||
|
|
|
|
|
|
|
| ||
| Loss before taxes |
|
| (3,987,018 | ) |
|
| (4,158,413 | ) |
| Expected income tax recovery |
|
| (856,424 | ) |
|
| (883,841 | ) |
| Non-deductible items |
|
| 200,573 |
|
|
| 8,544 |
|
| Change in estimates |
|
| 92,083 |
|
|
| 948 |
|
| Effect of changes in foreign and long-term tax rates |
|
| - |
|
|
| - |
|
| Change in valuation allowance |
|
| 566,087 |
|
|
| 892,013 |
|
| Total income taxes |
|
| 2,319 |
|
|
| 17,664 |
|
|
| Deferred taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes. Deferred tax assets at August 31, 2020 and 2019 are comprised of the following: |
|
|
| August 31 2020 $ |
|
| August 31 2019 $ |
| ||
| Non-capital losses |
|
| 5,588,526 |
|
|
| 5,022,440 |
|
| Marketable securities |
|
| 2,300 |
|
|
| 2,300 |
|
| Total unrecognized deferred tax assets |
|
| 5,590,826 |
|
|
| 5,024,740 |
|
|
| The Company has net operating loss carry-forwards of approximately $26,891,000 which may be carried forward to apply against future year income tax for U.S. tax purposes. |
| Year | Amount | Canada |
| 2025 | 76,000 | - |
| 2026 | 508,000 | - |
| 2027 | 1,056,000 | - |
| 2028 | 720,000 | - |
| 2029 | 753,000 | - |
| 2030 | 552,000 | - |
| 2031 | 538,000 | - |
| 2032 | 252,000 | - |
| 2033 | 344,000 | - |
| 2034 | 3,257,000 | - |
| 2035 | 1,934,000 | - |
| 2036 | 1,150,000 | - |
| 2037 | 1,857,000 | - |
| 2038 | - | - |
| 2039 | - | 242,000 |
| 2040 | - | 309,000 |
| Indefinite | 13,343,000 | - |
| 26,340,000 | 551,000 | |
| Total |
| 26,891,000 |
| 21. | Subsequent Events |
|
|
|
|
| September 22, 2020, Lexaria announced that U.S. Patent No. 10,756,180 was granted that provides patent claims that protect the use of Lexaria's DehydraTECH technology together with cannabinoids, nicotine, nonsteroidal anti-inflammatory drugs, or vitamins in mix and serve beverage formats. The patent is entitled “Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof”. |

| F-26 |
| Table of Contents |
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following is a statement of approximate expenses to be incurred by the Company in connection with the distribution of the securities registered under this registration statement. All amounts shown are estimates except for the SEC registration fee and FINRA filing fee.
|
|
| Amount |
| |
| SEC registration fee |
| $ | 2,609.67 |
|
| FINRA filing fee |
| $ | 3,398.00 |
|
| NASDAQ fee |
| $ | 50,000 |
|
| Legal fees and expenses |
| $ | 150,000 |
|
| Accountant’s fees and expenses |
| $ | 25,000 |
|
| Miscellaneous |
| $ | 23,500 |
|
| Total |
| $ | 254,507.67 |
|
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
The NRS empower us to indemnify our directors and officers against expenses relating to certain actions, suits or proceedings as provided for therein. In order for such indemnification to be available, the applicable director or officer must not have acted in a manner that constituted a breach of his or her fiduciary duties and involved intentional misconduct, fraud or a knowing violation of law, or must have acted in good faith and reasonably believed that his or her conduct was in, or not opposed to, our best interests. In the event of a criminal action, the applicable director or officer must not have had reasonable cause to believe his or her conduct was unlawful.
Pursuant to our articles, we may indemnify each of our present and future directors, officers, employees or agents who becomes a party or is threatened to be made a party to any suit or proceeding, whether pending, completed or merely threatened, and whether said suit or proceeding is civil, criminal, administrative, investigative, or otherwise, except an action by or in the right of the Company, by reason of the fact that he is or was a director, officer, employee, or agent of the Company, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise, against expenses, including, but not limited to, attorneys' fees, judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit, proceeding or settlement, provided such person acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interest of the Company, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
The expenses of directors, officers, employees or agents of the Company incurred in defending a civil or criminal action, suit, or proceeding may be paid by the Company as they are incurred and in advance of the final disposition of the action, suit, or proceeding, if and only if the director, officer, employee or agent undertakes to repay said expenses to the Company if it is ultimately determined by a court of competent jurisdiction, after exhaustion of all appeals therefrom, that he is not entitled to be indemnified by the corporation.
No indemnification shall be applied, and any advancement of expenses to or on behalf of any director, officer, employee or agent must be returned to the Company, if a final adjudication establishes that the person's acts or omissions involved a breach of any fiduciary duties, where applicable, intentional misconduct, fraud or a knowing violation of the law which was material to the cause of action.
| 96 |
| Table of Contents |
The NRS further provides that a corporation may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out of his status as such, whether or not the corporation has the authority to indemnify him against such liability and expenses. We have secured a directors’ and officers’ liability insurance policy. We expect that we will continue to maintain such a policy.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
On December 1, 2017, the Company issued 488 restricted shares of common stock at an issuance price of $24.59 per shares to settle $12,000 of accounts payable to a director of the Company.
On December 1, 2017, the Company granted 6,667 stock options with an exercise price of $24.90 and an expiration date of December 1, 2022 to an officer of the Company, pursuant to an existing management contract. The Company also granted 8,333 stock warrants with an exercise price of $24.90 and an expiration date of December 1, 2019 to a manager of the Company, pursuant to a management contract.
On December 1, 2017, the Company awarded a total of 6.969 restricted shares of common stock at an issuance price of $24.60 as required by intellectual property performance thresholds within an existing management consulting contract with the Company divided between three officers and three managers.
On January 17, 2018, 109 new warrants were issued to Haywood Securities related to the exercise of the broker warrants, each warrant good to buy one share of common stock at a price of 17.98 until April 3, 2019.
On May 25, 2018, we granted to Nuka 8,333 warrants expiring three years after issuance with an equity exercise price of $46.50 as required by a consulting contract.
On May 28, 2018, we announced that pursuant to our existing stock option plans, we granted stock options to directors, officers, employees and consultants that enable the option holders to purchase up to 57,500 shares of common stock at a price of $45.90 for a period of five years, vesting immediately.
On August 3, 2018, we issued 5,750 and 6,083 restricted shares of common stock at issuance prices of $37.20 and $39.60 respectively as required by executive consulting agreements upon certain intellectual property achievements, shared by the Chief Executive Officer and the President of the Company.
On August 31, 2018, we issued a total of 2,300 restricted shares of common stock at an issue price of $62.10 as required by executive consulting agreements to the Chief Executive Officer and the President of the Company. The shares are required to be issued upon certain intellectual property achievements and patent application filings in June that triggered the awards.
On January 25, 2019, we received $108,000 from the exercise of warrants to purchase an aggregate of 6,000 shares of common stock, at an exercise price of $18.00 per share, previously granted to third parties who are neither officers nor directors of the Company and have issued 6,000 shares of common stock as a result. We also issued 3,333 restricted shares of common stock in a transaction at an issue price of $39.30 as required by a consulting agreement.
| 97 |
| Table of Contents |
On November 13, 2019, we closed a non-brokered private placement offering under which we received $699,410.25 from the sale of 51,808 units at a price of $13.50 per unit to investors who are neither officers nor directors of the Company. Each unit is comprised of one share of common stock and one share purchase warrant whereby each share purchase warrant entitles the holder thereof to purchase an additional share for a period of two years, at an exercise price of $24.00 per share until November 13, 2020 and thereafter at a price of $36.00 per share until November 13, 2020. In connection with the issuance of the units, we also paid to certain finders an aggregate of $3,937.50 and issued warrants to purchase an aggregate of 292 shares of common stock having the same terms and conditions as those comprising part of the units.
On November 28, 2019, we closed the second tranche of our non-brokered private placement offering under which we received $121,275.00 from the sale of 8,983 units at a price of $13.50 per unit to investors who are neither officers nor directors of the Company. Each unit is comprised of one share of common stock and one share purchase warrant whereby each share purchase warrant entitles the holder thereof to purchase an additional share for a period of two years, at an exercise price of $24.00 per share until November 28, 2020 and thereafter at a price of $36.00 per share until November 28, 2020.
On May 6, 2020 and May 11, 2020, and in two separate tranches, we issued to certain investors an aggregate of 295,540 shares and warrants to purchase up to 295,540 shares for aggregate proceeds of $2,039,228. The warrants have a five-year term and may be exercised at $10.50 per share. As compensation for placement agent services provided in connection with the private placement, the Company issued to Bradley Woods & Co. Ltd. warrants to purchase up to 21,637 shares of common stock on the same terms as the warrants issued to the investors.
On June 29, 2020 we issued 11,574 shares of common stock to a consultant, bearing a deemed aggregate value of $100,000, or $8.64 per Share. The shares were issued as partial compensation for investor relations services to be provided to the Company
In connection with each of the foregoing issuances, the Company relied upon the exemption from registration provided by Section 4(a)(2) of the Securities Act of 1933, as amended, for transactions not involving a public offering and/or Rule 506 thereunder.
| ITEM 16. EXHIBITS |
(a) Exhibits
We have filed the exhibits listed on the accompanying Exhibit Index of this registration statement and below in this Item 16:
| 98 |
| Table of Contents |
| Exhibit Number |
| Description |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
| 4.4 ** |
| Form of Warrant Agency Agreement to be entered into between the Company , Computershare, Inc. and Computershare Trust Company, N.A., including the form of warrant |
|
| ||
| 10.1 |
| License Agreement dated August 11, 2015 with PoViva Tea LLC (incorporated by reference to exhibit 10.1 of Current Report on Form 8-K filed August 12, 2015) |
| 10.2* |
| Licensing Agreement dated May 14, 2016 of Lexaria Bioscience Corp. (incorporated by reference as exhibit 10.1 of our Current Report on Form 8-K filed May 20, 2016) |
| 10.11 |
| Form of Warrant issued October 31, 2018 |
| 99 |
| Table of Contents |
|
| ||
|
| ||
|
| ||
|
| ||
| 10.26 |
| Form of Warrant issued November 13, 2019 and November 28, 2019 |
|
| ||
|
| ||
|
| ||
| 23.2 |
| Consent of Sichenzia Ross Ference LLP (Included in Exhibit 5.1) |
| 24.1 |
| Power of Attorney (Included in the signature page to our Registration Statement on Form S-1 filed November 20, 2020) |
__________
| * | Confidential treatment was requested with respect to certain portions of this exhibit pursuant to 17.C.F.R. §240.24b-2. Omitted portions were filed separately with the SEC. |
| ** | To be filed by amendment. |
| 100 |
| Table of Contents |
| ITEM 17. UNDERTAKINGS |
The undersigned registrant hereby undertakes:
|
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
|
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
|
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
|
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
|
| (2) | That for the purpose of determining any liability under the Securities Act of 1933 each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
|
| (4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| 101 |
| Table of Contents |
|
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
|
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
|
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
|
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
|
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
|
| (6) | The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser. |
|
| (7) | Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the provisions described in Item 14 above, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
|
| (8) | The undersigned Registrant hereby undertakes: |
|
| (1) | That for purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4), or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
|
| (2) | That for the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and this offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| 102 |
| Table of Contents |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Kelowna, British Columbia, on the 6th day of January, 2021.
| LEXARIA BIOSCIENCE CORP. | |||
|
|
|
|
|
|
| By: | /s/ Christopher Bunka |
|
|
|
| Christopher Bunka |
|
|
|
| Chief Executive Officer and Chairman |
|
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature: |
| Capacity: |
| Date: |
|
|
|
| ||
| /s/ Christopher Bunka |
| Chief Executive Officer and Chairman |
| January 6, 2021 |
| Christopher Bunka |
| (Principal Executive Officer) |
|
|
|
|
|
| ||
| /s/ Allan Spissinger |
| Chief Financial Officer |
| January 6, 2021 |
| Allan Spissinger |
| (Principal Financial and Accounting Officer) |
|
|
|
|
|
| ||
| /s/ John Docherty* |
| President and Director |
| January 6, 2021 |
| John Docherty |
|
|
|
|
|
|
|
| ||
| /s/ Nicholas Baxter* |
| Director |
| January 6, 2021 |
| Nicholas Baxter |
|
|
|
|
|
|
|
| ||
| /s/ Ted McKechnie* |
| Director |
| January 6, 2021 |
| Ted McKechnie |
|
|
|
|
|
|
|
| ||
| /s/ Brian Quigley* |
| Director |
| January 6, 2021 |
| Brian Quigley |
|
|
|
|
|
|
|
|
|
|
| * By: /s/ Christopher Bunka |
| Attorney in fact |
| January 6, 2021 |
| Christopher Bunka |
|
|
|
|
| 103 |