
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
For the fiscal year ended
or
For the transition period from [____] to [____]
Commission file number
(Exact name of registrant as specified in its charter) |
| ||
State or other jurisdiction of incorporation or organization |
| (I.R.S. Employer Identification No.) |
|
|
|
| ||
(Address of principal executive offices) |
| (Zip Code) |
Registrant’s Telephone number, including area code:
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
| Name of Each Exchange On Which Registered |
N/A |
| N/A |
Securities registered pursuant to Section 12(g) of the Act:
Title of Class | Trading Symbol(s) | Name of each exchange on which registered |
Warrants | LEXXW | Nasdaq |
Indicate by check mark if the registered is a well-known seasonal issuer, as defined in Rule 405 the Securities Act Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act Yes ☐
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the last 90 days.
Indicate by check mark whether the registrant has submitted electronically, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-K (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ |
☒ | Smaller reporting company | ||
|
| Emerging growth company |
If an emerging growth company, indicate by a check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
As of February 28, 2021, the last day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the common stock held by non-affiliates of the registrant was approximately $
Indicate the number of shares outstanding of each of the registrant’s classes of common stock as of the latest practicable date.
DOCUMENTS INCORPORATED BY REFERENCE
None.
TABLE OF CONTENTS

| Page 2 of 90 |
| Table of Contents |
Cautionary Note Regarding Forward-Looking Statements
This annual report contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. Any statements contained herein that are not statements of historical fact may be forward-looking statements relating to future events or our future financial performance and are based on our present beliefs and assumptions as well as the information currently available to us. In some cases, you can identify forward-looking statements by terminology such as "may", “will”, "should", “could”, “targets”, “goal”, "expects", "plans", "anticipates", "believes", "estimates", "predicts", "potential" or "continue" or the negative of these terms or other comparable terminology.
These statements contain predictions and involve known and unknown risks, including the risks in the section entitled "Risk Factors" set forth in Item 1(A) in this report on Form 10-K, uncertainties and other factors that may cause our or our industry's levels of activity, performance, achievements, or actual results to be materially different from any future levels of activity, performance, achievements, or results expressed or implied by these forward-looking statements. Although we contend that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee levels of activity, performance, achievements, or future result.
Forward-looking statements in this report include statements about, among other things: the status, progress and results of our research programs; our ability to obtain regulatory approvals for, and the level of market opportunity for, our product candidates; our business plans, strategies and objectives, including plans to pursue collaboration, licensing or other similar arrangements or transactions; our expectations regarding our liquidity and performance, including our expense levels, sources of capital and ability to maintain our operations as a going concern; the competitive landscape of our industry; and general market, economic and political conditions
We caution you not to place undue reliance on any forward-looking statements as they speak only as of the date on which such statements were made, and we do not assume any obligation to update any forward-looking statement or to reflect the occurrence of an unanticipated event. New factors may emerge and it is not possible to predict all factors that may affect our business and prospects. Further, management cannot assess the impact of each factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
Solely for convenience, tradenames and trademarks referred to in this Annual Report on Form 10-K appear without the ® or TM symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to these tradenames or trademarks, as applicable. All tradenames, trademarks, and service marks included or incorporated by reference in this Annual Report on Form 10-K are the property of Lexaria Bioscience Corp.
As used in this report, the terms "Lexaria" "we", "us", "our" and "Company" mean Lexaria Bioscience Corp. and/or our subsidiaries, unless otherwise indicated.

| Page 3 of 90 |
| Table of Contents |
PART 1
Item 1. Business
General and Historical Overview of Our Business
Lexaria is a biotechnology company seeking to enhance the bioavailability of a broad variety of active pharmaceutical ingredients (“APIs”) with its DehydraTECHTM drug delivery technology. DehydraTECH combines lipophilic APIs with specific fatty acid and carrier compounds thereby improving the way APIs enter the bloodstream while increasing the effectiveness of fat-soluble active molecules allowing lowering overall dosing and promoting healthier oral ingestion methods. DehydraTECH can be used with a wide variety of APIs encompassing fat-soluble vitamins, non-steroidal anti-inflammatory drugs (“NSAIDs”) pain medications, hormones, phosphodiesterase inhibitors, antivirals, nicotine and its analogs, and all cannabinoids including tetrahydrocannabinol (“THC”) for a variety of therapeutic indications, including hypertension, SARS-CoV-2/COVID-19 and HIV/AIDS. The Company’s technology applies to a host of different ingestible or topically administered product formats including foods, beverages, oral suspensions, tablets, capsules, creams, lotions, and skin patches.
Lexaria began filing patents for DehydraTECH in 2014 with two initial US provisional patent application filings by the original inventors Poppy’s Teas LLC, which Lexaria acquired by way of exclusive, worldwide license rights and controlling interest in the founding company. We have since increased the number of patent applications to approximately 60 with 23 patents granted worldwide to date. In addition to the US patent filings, the Company has also pursued international patent protection through filings under the Patent Cooperation Treaty, followed by national filings in over 40 jurisdictions of highest commercial potential thereunder. Our patent family includes intellectual property addressing the manufacturing and processing methods used to combine the long chain fatty acids with active pharmaceutical ingredients.
Lexaria’s patent applications developed from its Research and Development programs (“R&D”) currently include fat-soluble versions of vitamins, NSAIDs, nicotine, cannabinoids, hormones, phosphodiesterase inhibitors, and antivirals. 2018 animal studies demonstrated a propensity for DehydraTECH technology to elevate the quantity of drug delivered across the blood-brain-barrier. This expanded our patent applications and opened possibilities for improved delivery of certain central nervous system-targeted drugs that require additional R&D.
In a human clinical study performed in 2018 and published in 2019 in a peer reviewed medical journal, Advances in Therapy titled “Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study” available on the PubMed.gov website with the identification of PMID: 31512143, Lexaria demonstrated that its technology delivered higher volumes of cannabidiol into the human circulatory system and did so more quickly than a concentration-matched positive control. This same study also demonstrated a statistically significant reduction in human blood pressure from the DehydraTECH processed cannabidiol, versus no statistical reduction in human blood pressure from the positive control.
We operate a Health Canada-licensed laboratory in Canada to conduct basic research and formulation operations, and typically outsource virtually all analytical work to independent third-party laboratories located in Canada, the USA, and Europe. Such third-party evaluation provides independent confirmation of the effects of our technology and processes.

| Page 4 of 90 |
| Table of Contents |
Lexaria’s formulation and process-oriented operations are primarily conducted in its own laboratory and validated through third-party testing, in preparation for partnering with industry leaders for adoption into their consumer products and/or drugs. Other than for R&D purposes, Lexaria does not produce, manufacture, market or distribute drugs.
Although we have experimented with consumer product development in the past, those activities occupy a declining amount of our corporate time. We first began selling trial amounts of ViPova branded black tea fortified with hemp oil and utilizing our technology, in January 2015 and added additional flavours over time.
We also began offering our first coffee and hot chocolate also fortified with full spectrum hemp oil, and also under the ViPova brand. Beginning in January 2021 we discontinued sales of consumer products, but offering a variety of self-made beverages to consumers helped us to establish the ViPova brand and helped us to develop final consumer product formulations and understand consumer needs
Generating meaningful revenue from consumer product sales was challenging and we were unable to achieve widespread retail distribution. We continue to be open to the possibility of generating sales from international markets, in those locations where hemp oil fortified foods are permissible by law.
ViPova branded products are owned by our wholly owned PoViva Corp. subsidiary. Lexaria Energy, TurboCBD and ChrgD+ branded products are owned 100% by Lexaria Bioscience Corp.
Through our product development we have communicated to the industry the versatility of our technology in specific CPG formats and we believe this strategy has been successful in assisting us in technology licensing discussions with potential new clients. We believe the range of products available and under development are sufficient to prepare for revenue growth and potentially profitable long-term operations if we are able to generate sufficient business clientele demand.
Our business strategy contains an element that we believe will be more impactful to future corporate growth that involves the further development and out-licensing of our intellectual property of molecule delivery that enhances bioactivity or absorption. We have no plans to offer for sale any products containing THC in quantities higher than 0.3%. We have discontinued all direct business activities related to non-FDA-approved uses of THC, including our former business practice of licensing our technology to businesses that were legally state-licensed to offer THC products. We also plan to license our technology to other companies for the delivery of molecules other than THC or cannabinoids, such as nicotine which we have licensed to Altria Ventures Inc., an indirect wholly owned subsidiary of Altria Group, Inc. Our October 31, 2017, announcement of the USPTO Notice of Allowance for our first patent granted and the subsequent granted patents of our technology in the US and in many other countries around the world related to new molecule groups, along with our ongoing patent filing and grants, may enhance our ability to successfully pursue our licensing initiatives during fiscal 2022.
We continue to communicate the benefits of our technology to potential licensing partners; i.e. with higher absorption levels a manufacturer could perhaps infuse smaller amounts of active molecules into a product, potentially reducing their manufacturing input costs; to provide higher bioavailability with the dosing limits being imposed or contemplated in many jurisdictions; to infuse beverages while masking the flavor and smell of the active molecules; and to reduce delivery times to the bloodstream. We believe these to be meaningful competitive advantages that may lead to the potential to generate licensing revenue, and will pursue these opportunities within the cannabinoids, nicotine, and other bioactive molecular markets both within the USA and also internationally, in those locations where they are legal and regulated by government.
Subject to budgetary availability, we also plan to conduct additional in vitro and in vivo studies testing the absorption of many API’s – CBD, NSAIDs, vitamins, PDE5 inhibitors, antiviral drugs, nicotine, and others– to substantiate the effectiveness of our technology. More than simply satisfying scientific curiosity, successful tests could lead to increased awareness and acceptance of our technology as a meaningful method by which to deliver some or all of the named molecules more effectively than their current delivery methods. Therefore, absorption tests could become an important element leading towards higher rates of acceptance of our technology licensing initiatives.

| Page 5 of 90 |
| Table of Contents |
We will pursue technology licensing opportunities as a method of generating highly profitable revenue streams over long periods of time. In addition, while nine of our US patents and eight of our Australian patents have been granted to date, we now have received granted patents in the European Union, Japan, India and Mexico, and have multiple other applications filed in the US and around the world. It is not possible to forecast with certainty when, or if, our remaining patents pending will become granted patents. But if our remaining patent applications do become granted patents, our ability to generate meaningful license revenue from our intellectual property may increase from multiple jurisdictions outside of the US.
We will continue to pursue our remaining patents pending as vigorously as we are able, since the successful granting of more of those applications could lead to material increases in shareholder value. We are pursuing patent protection in more than 40 countries around the world.
Available Information
The address of our principal executive office and research laboratory is #100–740 McCurdy Road, Kelowna, British Columbia, Canada V1X 2P7.
Our common stock is quoted on the Nasdaq under the symbol “LEXX”. We file annual, quarterly, and current reports, proxy statements and other information with the U.S. Securities Exchange Commission (the “SEC”). These filings are available to the public on the Internet at the SEC’s website at http://www.sec.gov.
Our corporate website is located at www.lexariabioscience.com (this website address is not intended to function as a hyperlink and the information contained on our website is not intended to be a part of this Report). We make available free of charge on https://www.lexariabioscience.com/investors/regulatory-filings/ our annual, quarterly, and current reports, and amendments to those reports if any, as soon as reasonably practical after we electronically file such material with, or furnish it to, the SEC. We may from time to time provide important disclosures to investors by posting them in the Investor Relations section of our website.
We maintain our registered agent's office and our U.S. business office at Nevada Agency and Transfer Company, 50 West Liberty, Suite 880, Reno, Nevada 89501. Our telephone number is (755) 322-0626.
Lexaria Bioscience Corp. is a British Columbia based reporting issuer in Canada and as such, we are required to file certain information and documents at www.sedar.com.
Our Current Business
Our business plan is currently focused on the development of strategic partnerships with licensees for our patented DehydraTECH technology in exchange for up front and/or staged licensing fees and/or royalty payments over time.
We continue to investigate national and international opportunities to investigate expansions and additions to our intellectual property portfolio. Patents have been filed specifically for the use of DehydraTECH with cannabinoids for the treatment of heart disease.
We plan to perform additional human clinical investigations in late calendar 2021 and throughout 2022 related to enhanced DehydraTECH formulations of cannabidiol in pre- and mildly hypertensive middle-aged subjects to gather additional information on blood pressure reduction potential. Lexaria also plans to conduct during fiscal 2022, evaluations of DehydraTECH’s ability to improve the oral delivery characteristics and pharmacological performance of certain other APIs. We will continue to seek beneficial acquisitions of intellectual property if and when we believe it is advisable to do so.

| Page 6 of 90 |
| Table of Contents |
Our current patent portfolio includes patent family applications or grants pertaining to Lexaria’s method of improving bioavailability and taste, and the use of DehydraTECH as a delivery platform for a wide variety of Active Pharmaceutical Ingredients (“APIs”) encompassing all cannabinoids including tetrahydrocannabinol (“THC”); fat soluble vitamins; NSAIDs pain medications; and nicotine and its analogs.
Lexaria hopes to reduce common but less healthy administration methods, such as smoking cigarettes as a delivery method for nicotine, by way of enabling development of safe and effective oral nicotine dosage forms through licensing arrangements with major tobacco companies, as it demonstrates the benefits of DehydraTECH for public health. The Company is aggressively pursuing patent protection in jurisdictions around the world. The Company currently has more than 50 patent applications pending worldwide, with 23 patents granted to date. Due to the complexity of pursuing patent protection, the quantity of patent applications will vary continuously as each application advances or stalls. Lexaria is also filing new patent applications for new discoveries that arise from the Company’s R&D programs and, due to the inherent unpredictability of scientific discovery, it is not possible to predict if or how often such new applications might be filed.
During the past fiscal year, the Company experienced the following significant corporate developments:
During the past fiscal year, the Company was granted an aggregate of four new patents in the following jurisdictions, Europe, India and Japan.
On December 9, 2020, CanPharm completed a disposition to Hill Street Beverage Company Inc. (“Hill Street”) of its use and licensing rights to use its DehydraTECH technology specifically in association with non-pharmaceutical products containing cannabis molecules that contain 0.3% or greater THC.
On January 11, 2021, Lexaria effected a reverse stock split that was conducted on a 1-for-30 basis on the Company’s issued share capital and on any outstanding warrants and options, whereby the exercise prices of such outstanding convertible securities were adjusted accordingly.
On January 12, 2021, Lexaria became a Nasdaq listed company and announced the pricing of a public offer of 1,828,571 units, with each unit comprising one share of common stock and one warrant to purchase one share of common stock at $5.25 per unit. The warrants issued pursuant to this public offering were listed on the Nasdaq under the symbol LEXXW and have an exercise price of $6.58 per share. They are immediately exercisable, and expire five years from issuance date. The underwriter was granted 30-day option to purchase up to an additional 274,285 shares of common stock and/or warrants to purchase up to the same amount of common stock, which option was exercised in full by H.C. Wainwright & Co. who acted as sole book-running manager for the offer. Gross proceeds of $11.04 million were ultimately received from the offering and Lexaria also issued five-year warrants to H.C. Wainwright & Co. entitling them to purchase up to 166,781 shares of common stock with an exercise price of $6.58 per share. Pursuant to certain tail rights held by Bradley Woods & Co. Lexaria paid Bradley Woods $316,999.62 and issued Bradley Woods five-year warrants to purchase 60,385 shares of common stock at an exercise price of $6.58 per share.
On January 14, 2021, Mr. Al Reese, Jr., was appointed to Lexaria’s board of directors. With the appointment of Mr. Reese Jr. Lexaria established a fully independent audit and finance committee and comply with the financial expert requirements of the Nasdaq.
On March 24, 2021, Lexaria announced results from a shelf-stability study. DehydraTECH CBD beverages demonstrated 93.4% of target CBD potency a year after production. The beverages also exhibited zero microbial growth over the period. Furthermore, the samples had intra-beverage variance less than 1% in CBD potency across various fractions (top, middle, and bottom) without mixing or agitation, indicating a very stable emulsion.

| Page 7 of 90 |
| Table of Contents |
On April 15, 2021, Lexaria announced the appointment of Gregory Downey as Chief Financial Officer, to replace outgoing Allan Spissinger whose contract ended on May 31, 2021.
During the spring of 2021, Lexaria commenced its human clinical study HYPER-H21-1 of DehydraTECH CBD, which is intended to validate DehydraTECH CBD’s effect on hypertension, and is a randomized, double-blind, controlled study expected to enroll 24 subjects with symptoms of either pre-hypertension or mild hypertension. A single 300 mg dose of DehydraTECH 2.0 CBD formulation will be compared against a non-DehydraTECH control of matched concentration. Time series blood pressure and heart rate analyses are primary objectives of the study. Secondary objectives include pharmacokinetic speed and rate of absorption of CBD and main metabolites as well as assessment of inflammatory markers of cardiovascular disease and nitric oxide biomarkers.
On July 5, 2021, the Company announced that it would, effective on market close July 7, 2021, voluntarily delist from the Canadian Securities Exchange (“CSE”) since an overwhelming majority of trading has moved to the Nasdaq resulting in saving management time, effort, and fees.
On June 7, 2021, Lexaria provided an update on HYPER-H21-1 progress, stating that 24 volunteers, ranging in age between 45 to 65, were dosed and the treatment was well tolerated with no serious adverse events or side effects observed or reported. The early results of this study were disclosed in July 2021 noting a difference between the DehydraTECH-CBD formulation and the control arm at the 20-minute mark was statistically significant at the 2.5% level.
Lexaria commenced a subsequent human trial study, designated as HYPER-H21-2, and completed its patient dosing in late July 2021. Initial results from the trial were disclosed subsequent to the year end. HYPER-H21-2 evaluated 16 volunteers who were pre or mildly hypertensive and received three separate doses of 150mg DehydraTECH 2.0 CBD versus placebo. HYPER-H21-2 concentrated on monitoring blood pressure reduction continuously over 24 hours and studying central arterial stiffness, physical activity and sleep quality.
The Company experienced the following significant corporate developments subsequent to August 31, 2021
Subsequent to the August 31, 2021 fiscal year, Lexaria was granted an additional two patents, one in Japan and our first in Mexico.
On September 7, 2021, Lexaria announced partial results from its human clinical study HYPER-H21-2 which evaluated DehydraTECH processed CBD in a 24-hour study of volunteers with mild to moderate hypertension. At selected times during the 24-hour study, volunteers with mild to moderate hypertension averaged as much as a 20 mmHg (i.e., 23%) decrease in blood pressure relative to placebo and over the 24-hour ambulatory monitoring period, volunteers averaged a significant reduction of 7.0% (p < 0.001) in systolic pressure with DehydraTECH-CBD relative to placebo.
On September 8, 2021, Lexaria announced that it had commenced the process for preparing an Investigational New Drug application for the purposes of filing same with the Food and Drug Administration with respect to registering its DehydraTECH-processed CBD as a pharmaceutical treatment for hypertension.
On October 5, 2021, Lexaria announced results from its oral nicotine absorption study NIC-A21-1 which revealed that DehydraTECH-nicotine delivered via the oral pouch product format required only 2 to 4 minutes to deliver nicotine levels in blood plasma comparable to levels achieved at 45 minutes with concentration-matched controls. DehydraTECH-nicotine also reached statistically significant peak blood plasma levels up to 10-fold higher overall than controls (p=0.004) while still clearing from blood virtually as quickly as the controls.

| Page 8 of 90 |
| Table of Contents |
On October 13, 2021, Lexaria announced the that its oral tetrahydrocannabinol (“THC”) absorption study THC-A21-1 revealed that DehydraTECH-THC delivered, via oral ingestion, required only 15 minutes to deliver THC levels in blood plasma comparable to levels achieved at 45 minutes with concentration-matched controls.
During the study DehydraTECH-THC delivered more THC into the bloodstream than the industry standard medium chain triglyceride (“MCT” or “coconut oil”) based control formulation from the 2-minute mark onwards, then dropped rapidly to the same level as the MCT control by the 6-hour mark.
On November 1, 2021, Lexaria commenced its first animal study EPIL-A21-1 to determine if DehydraTECH-CBD evidences superior treatment of seizure activity when compared to generic cannabidiol and Epidiolex and subsequently announced the following new research studies for the 2022 year:
| · | HYPER-H21-4: This 6-week efficacy study of approximately 60 volunteers who suffer from hypertension, will provide extensive data to Lexaria on how DehydraTECH-CBD treats hypertension and may provide additional long-term health benefits, including its effects on 24-hour ambulatory blood pressure; arterial stiffness and autonomic balance; brain structure and function through brain magnetic resonance imaging; blood biomarkers (including lipids such as cholesterol and more); renal, hepatic, sleep quality / daytime sleepiness / sleep disorders; actigraphy, geriatric depression scale, perceived stress, and Beck anxiety inventory. |
|
|
|
| · | HOR-A22-1: This PK study will evaluate the ability of DehydraTECH to enhance the delivery characteristics of estrogen. Estrogen helps to control the menstrual cycle but also controls cholesterol and protects bone health. |
|
|
|
| · | DEM-A22-1: This efficacy study will evaluate DehydraTECH-CBD with and without nicotine for the potential treatment of dementia. Alzheimer’s disease is the most common form of dementia and accounts for at least 60% of all cases, and nicotine is already showing promising results related to Alzheimer’s treatment. |
|
|
|
| · | RHEUM-A22-1: This efficacy study will focus on the ability of DehydraTECH-CBD to potentially affect treatment of rheumatoid disease. Given CBD’s postulated efficacy related to inflammation, Lexaria will explore a possible role for CBD in this area of investigation. Rheumatic diseases are autoimmune and inflammatory diseases that cause the immune system to attack joints, bones, muscles, and organs. |
Science and Technology
Lexaria is a drug delivery R&D company focused on developing and out licensing DehydraTECH for improved consumer experiences, rapidity, and delivery of bioactive compounds in oral and topical products. The Company is focusing its capital and management time on its pursuit of intellectual property, technology licensing opportunities, and an expanding portfolio of patent pending applications.
In 2014, the Company acquired the IP that formed our first patent application that was filed in the same year. From that first patent application, due to ongoing R&D investigation and work by Company management, we now have approximately 50 patent applications pending around the world, with 23 allowed/patents granted. All of our applications and allowed/granted patents relate to DehydraTECH and its enhancement of certain characteristics of oral ingredient and drug delivery. Additional early-stage investigation has been conducted of topically-administered products such as patches, creams and lotions.

| Page 9 of 90 |
| Table of Contents |
The Company developed a variety of demonstration products beginning in 2015 to demonstrate the potential uses for DehydraTECH to both consumers and potential licensees. These included teas, coffee, and protein energy bars – all utilizing DehydraTECH for the more palatable and efficient delivery of cannabinoids. The Company subsequently developed additional demonstration products including powder filled capsules and mix and serve powders for beverage incorporation also utilizing DehydraTECH for the more palatable and efficient delivery of bioactive molecules. The Company gained extensive experience and knowledge from the formulation and production of these demonstration products that facilitates assisting our licensees with the integration of DehydraTECH in their products.
In the production of our intermediate products for product manufacturers to use, each raw material, intermediate stage and completed product is assessed for compliance with all applicable regulations, and to ensure that the inputs and the finished products meet all applicable legal and quality standards including and as it relates to content; molds and mildews; heavy metals; and additional components.
The US Federal Government, through the US Department of Health and Human Services, owns US Patent #6630507, which among other things, claims that
“Cannabinoids have been found to have antioxidant properties, unrelated to NMDA receptor antagonism. This new found property makes cannabinoids useful in the treatment and prophylaxis of wide variety of oxidation associated diseases, such as ischemic, age-related, inflammatory and autoimmune diseases. The cannabinoids are found to have particular application as neuroprotectants, for example in limiting neurological damage following ischemic insults, such as stroke and trauma, or in the treatment of neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease and HIV dementia.”
For reference, cannabinoids are compounds that affect cannabinoid receptors located on many human cells. CB1 receptors are widely found within the human brain; and CB2 receptors are found with the human immune system and have been linked to anti-inflammatory and other responses.
Over one hundred different cannabinoids have been isolated from the cannabis plant, most of which do not have psychoactive properties. One that does have psychoactive properties is THC. Endocannabinoids are produced naturally in the human body while phyto cannabinoids are produced in several plant species, most abundantly in the cannabis plant.
Cannabidiol (“CBD”) is one of the major phyto cannabinoid and is not psychoactive, often comprising more than 35% of the extracts from the cannabis plant resin. CBD occurs naturally in other plant species beyond cannabis. For example, the most widely acknowledged alternative source of phyto cannabinoid is in the better understood Echinacea species, in widespread use as a dietary supplement. Most phyto cannabinoids are virtually insoluble in water but are soluble in lipids and alcohol. The World Anti Doping Agency (“WADA”) has exempted CBD from its 2018 list of banned substances.
In the U.S., the 2018 Farm Bill permits hemp cultivation and allows the transport of hemp-derived products across state lines, within a tightly regulated framework. Primary among these, the plant must contain less than 0.3% THC, and state departments of agriculture must submit their plans to license and regulate hemp to the Secretary of the USDA, or otherwise comply with a federally run hemp program. Legislative reform regarding CBD from hemp is continually evolving.

| Page 10 of 90 |
| Table of Contents |
Status of Operations
Most of Lexaria’s revenues are generated from third party businesses either licensing the intellectual property associated with DehydraTECH for incorporation into their products or purchasing DehydraTECH infused intermediate product as a raw material for use within their own products.
Intellectual Property
Since our first patent filing in 2014 for DehydraTECH, we have increased the number of patent applications to approximately 50 and to date have been allowed/granted 23 patents worldwide as of the date of this filing.
The substance of the patents center on the use of DehydraTECH in a variety of products including those that are ingested or topically administered such as CBD, food, beverage, patches, creams, lotions et cetera. Patents have been filed (and granted in both Australia and the EU) specifically for the use of DehydraTECH with cannabinoids for the treatment of heart disease. The pending and granted patents also cover the manufacturing and processing methods used to combine fatty acids with active pharmaceutical ingredients. This includes heating and drying methods and use of excipients and substrates. Below we summarize Lexaria’s allowed/granted patents.
Issued/Allowed Patent # | Patent Family |
US 9,474,725 B1 | Food and Beverage Compositions Infused with Lipophilic Active Agents and Methods of Use Thereof
|
US 9,839,612 B2 | |
US 9,972,680 B2 | |
US 9,974,739 B2 | |
US 10,084,044 B2 | |
US 10,103,225 B2 | |
US 10,381,440 | |
US 10,374,036 | |
US 10,756,180 | |
AU 2015274698 | |
AU 2017203054 | |
AU 2018202562 | |
AU 2018202583 | |
AU 2018202584 | |
AU 2018220067 | |
EP 3164141 | |
JP 6920197 | |
AU 2016367036 |
Methods for Formulating Orally Ingestible Compositions Comprising Lipophilic Active Agents |
JP 6963507 | |
MX 011399 | |
AU 2016367037 | Stable Ready-to-Drink Beverage Compositions Comprising Lipophilic Active Agents |
IN 365864 | |
JP 6917310 |

| Page 11 of 90 |
| Table of Contents |
On June 11, 2015, Lexaria initiated the simultaneous filing of a U.S. utility patent application and an international patent application under the Patent Cooperation Treaty (PCT) procedure, both through the U.S. Patent and Trademark Office (“USPTO”). These applications follow the Company’s 2014 and 2015 family of provisional patent application filings in the U.S. and serve two additional broad purposes:
| · | Lexaria is seeking protection of its intellectual property under international treaties. To this end Lexaria has filed for PCT patent application protection. There are 148 countries that are signatories to the Patent Cooperation Treaty, including such major markets as Canada, China, India, much of Europe and the Middle East, the United Kingdom and Japan among others. |
|
|
|
| · | Lexaria has demonstrated that its lipid infusion technology has applications beyond the delivery of just cannabinoids. Based on further formulation testing, Lexaria has included additional lipophilic molecules that may be delivered via oral administration utilizing its technology, widely encompassing three major market opportunities for the Company: Nicotine; NSAIDs; and Vitamins. |
In December 2015, the Company filed two further provisional patent applications in the U.S. These new applications served to further broaden the variety and applicability of base compounds that can be used when formulating DehydraTECH. The first of these applications identify compounds like edible starches (e.g., tapioca starch) that are commonly used in oral and pharmaceutical products today and could, therefore, serve as a base for formulating and incorporating DehydraTECH into a wide variety of products. The second of these applications identify emulsifier compounds like gum arabic that are commonly used in beverage products today in order to facilitate similar flexibility for formulating DehydraTECH in shelf-stable beverages.
On October 26, 2016, the USPTO issued U.S Patent No. 9474725, Food and Beverage Compositions Infused with Lipophilic Active Agents and Methods of Use Thereof, pertaining to our method of improving bioavailability and taste of certain cannabinoid lipophilic active agents in food products. This was the Company’s first patent granted and has a publish date of October 27, 2016 (June 15, 2017, in Australia No. 2015274698) and protects DehydraTECH for twenty years. Additional patent grants include, but are not limited to the use of DehydraTECH as a delivery platform, “composition of matter” claims that protect the specific combination of substances which enable improved taste and bio absorption properties, that protect processes for making specific compositions of matter for enhanced cannabinoid delivery utilizing DehydraTECH. Of note, Lexaria has received issuance of patents in its second and third patent families representing the first time the Company has been granted claims for use of DehydraTECH in connection with the treatment of specific diseases and medical conditions affecting humans, which the Company believes will prove to be of significance to the pharmaceutical industry sector as it further develops and grows.
International Patent Protection
Lexaria first began work in the fields of enhanced delivery of active ingredients and drugs in 2014 focusing our efforts on R&D within the U.S. and Canadian marketplaces with our demonstration products to licence DehydraTECH to product manufacturers. Our pursuit and development of our technology has expanded our potential area of impact, both geographically and by sector. Because of the applicability of DehydraTECH to many market sectors across the globe, we have taken the necessary steps to protect that intellectual property internationally.
Additional Molecules
Lexaria does not intend to create or produce consumer products ourselves, rather, our business plan is to encourage existing participants within these sectors to license and utilize DehydraTECH to enable enhanced performance of their products across a wide range of lipophilic bioactive molecules of interest to us including and beyond CBD. Some of these additional lipophilic bioactive molecules of interest are summarized below, and additional molecules of interest are continually being evaluated.

| Page 12 of 90 |
| Table of Contents |
Antivirals.
Viruses and bacteria cause the most common infectious diseases in the world today. Vaccines can offer protection against contracting viral and bacterial infections, whereas antiviral drugs and antibiotics respectively are required as treatments to combat disease if vaccination or other protective measures are inadequate or are not available. Early research findings have shown that some known antiviral drugs like remdesivir, interferon beta-1b, lopinavir, ritonavir and ribavirin among others, evaluated alone and in combination treatment regimens, may have utility against COVID-19 caused by infection with the novel coronavirus. Most of the antiviral drugs currently available are used to treat infections caused by HIV, herpes viruses, hepatitis B and C viruses, and influenza A and B viruses, and are therefore being repurposed to evaluate prospective utility against COVID-19. While a host of antiviral drugs exist or are under development today as treatments for COVID-19 and other infectious disease conditions, many of them are hindered by poor water solubility which, in turn, results in their poor absorption and uptake by the body if taken orally, frequently limiting their overall therapeutic effectiveness. To attempt to overcome this, oral antiviral medications often have to be given at high doses which can result in a variety of unwanted side effects including diarrhea, headache, nausea, vomiting, stomach upset, drowsiness, dizziness, vision changes, difficulty breathing and other bodily dysfunctions. Alternatively, in some cases it is necessary to administer antiviral medications by way of needle injection for easier access to the bloodstream circumventing the gastrointestinal absorption limitations as is the case with, for instance, remdesivir, as mentioned above. However, injectable administration requires involvement of a medical practitioner which may not be easily accessible for the masses, usually increases cost of a medicine and often means that the product format isn’t as stable or requires special storage and handling considerations relative to oral medications.
Nicotine.
More than 99% of all nicotine consumed worldwide is delivered through smoking cigarettes. Approximately 6,000,000 deaths per year, worldwide, are attributed primarily to the delivery of nicotine through the act of smoking according to the Centers for Disease Control and Prevention, which also estimates that over $170 billion per year is spent just in the U.S. on direct medical care costs for adult smokers. 69% of U.S. adult smokers want to quit smoking and 43% of U.S. adult smokers have attempted to quit in any twelve-month period.
Worldwide, legal retail cigarette sales were worth US$814 billion in 2018 with illegal sales thought to represent another 11.2% of the global market (bat.com) with over 5.3 trillion cigarettes sold to more than 1 billion smokers.
Non-steroidal anti-inflammatories.
NSAIDs are the second-largest category of pain management treatment options in the world and are used both for pain management and for treatment of inflammation. The anti-inflammatory therapeutic market is expected to generate $106.1 billion in 2020, globally (alliedmarketresearch.com). Incurable inflammatory autoimmune diseases included arthritis, asthma, and chronic obstructive pulmonary disease (COPD). The U.S. makes up over one-half of the global market. The opioids market (such as morphine) forms the largest single pain management sector but are known to be associated with serious dependence and tolerance issues.
Some of the most commonly known NSAIDs are ASA (Aspirin), Ibuprofen (Advil, Motrin), and Acetaminophen (Tylenol - Acetaminophen is not accepted by all persons to be an NSAID). Although NSAIDs are generally a safe and effective treatment method for pain, they have been associated with a number of gastrointestinal problems including dyspepsia and gastric bleeding and certain adverse effects on human kidneys.
On August 11, 2015, Lexaria signed a license agreement with PoViva Tea LLC for $10,000, granting Lexaria a 35-year non-exclusive worldwide license to unencumbered use of PoViva Tea LLC’s IP Rights, including rights of resale. This license agreement ensures Lexaria has full access to the underlying infusion technology. On January 14, 2019, this agreement was updated whereby PoViva Corp. (formerly PoViva Tea LLC) granted Lexaria an exclusive license to use DehydraTECH technology for a period of time ending 25 years after the date of the last patent granted to PoViva Corp.

| Page 13 of 90 |
| Table of Contents |
Scientific testing and validation
CBD and Other Cannabinoid Programs
Our experimentation with, and validation of DehydraTECH technology has been ongoing since 2015. On August 24, 2015, the Company announced achievements in enhanced gastro-intestinal absorption of CBD utilizing DehydraTECH. The third-party testing was conducted in two phases of in vitro tests beginning in June and completed in August 2015.
The independent laboratory results delivered average CBD permeability of 499% of baseline permeability, compared to CBD permeability without DehydraTECH, exceeding Company expectations. This was assessed in a strictly controlled, in vitro experiment using a human intestinal tissue model.
The tests also showed 325% of baseline gastro-intestinal permeability of CBD comparing Lexaria’s CBD-fortified ViPova black tea to a second control of CBD and black tea combined, without Lexaria’s patented formulation enhancements. This confirmed that the specialized processing undertaken by Lexaria during its manufacturing process together with its formulation enhancements, does indeed significantly improve absorption levels.
The bioavailability of CBD (or of THC) varies greatly by delivery method. Smoking typically delivers cannabinoids at an average bioavailability rate of 30% (Huestis (2007) Chem. Biodiverse. 4:1770–1804; McGilveray (2005) Pain Res. Manag. 10 Suppl. A:15A – 22A). By comparison, orally consumed cannabis edibles typically deliver cannabinoids at an average bioavailability rate of only 5% (Karschner et al. (2011) Clin. Chem. 57:66–75).
During January 2015, Lexaria conducted a study of nitric oxide levels in humans, as a biomarker for absorption of CBD, with the expectation that it would provide additional evidence of the efficient absorption of CBD from DehydraTECH -enhanced oral products enhanced with hemp oil, by demonstrating the elevation of nitric oxide in the human body in response to oral ingestion.
The study data from human subjects demonstrated significant elevation of systemic nitric oxide levels as a surrogate biomarker for CBD bio absorption in response to ingestion of Lexaria’s oral delivery. This provided clinical support for the CBD bioavailability enhancing properties of DehydraTECH, on the premise that bioavailable CBD is known to elevate levels of the endocannabinoid anandamide in the human body which, in turn, stimulates release of nitric oxide in the vascular system.
In August of 2018 we released the results from our randomized, placebo-controlled, double-blind European human clinical study that evaluated a DehydraTECH-CBD hemp oil capsule developed by Lexaria. The degree and speed of CBD absorption into blood plasma and potential cardiovascular and cognitive performance enhancement in 12 healthy male volunteers was studied.
Key metabolic and hemodynamic performance findings linked to bioavailability enhancements were revealed in the study as released in February 2019, which compared a 90 mg dose of Lexaria’s DehydraTECH enhanced TurboCBD capsules to a 90 mg dose without DehydraTECH (the “positive control”) as well as a placebo, as follows:
| · | Analysis of mean arterial blood pressure (MAP) at peak blood levels of CBD achieved with Lexaria’s TurboCBD demonstrated a significant reduction in MAP compared to placebo (95% CI; p=0.027). This finding was not observed with the dose-matched positive control formulation for which there was no significant decrease in MAP compared to placebo (95% CI; p=0.625); |
|
|
|
| · | Cerebral perfusion was also analysed by an index of conductance in the middle cerebral artery (MCA). The findings revealed that Lexaria’s TurboCBD caused the greatest increase in MCA conductance relative to both the positive control formulation and placebo (95% CI; p=0.017 and P=0.002 respectively); |

| Page 14 of 90 |
| Table of Contents |
Finally, over the six-hour study, analysis of the total area under the curve (AUC) demonstrated that Lexaria’s DehydraTECH enhanced TurboCBD capsules resulted in a notable trend for higher levels of CBD in the bloodstream overall than the positive control formulation with total AUC of 10,865 ± 6,322 observed with Lexaria’s formulation compared to 7,115 ± 2,978 observed with the positive control (95% CI; p=0.096).
These results corroborate and confirm other in vitro and in vivo studies that evaluated DehydraTECH. Although this study evaluated absorption only of CBD and its metabolites, Lexaria believes nearly identical bioavailability enhancement results would be achieved with other cannabinoids.
During March of 2019 we also launched an in vivo research program to test Lexaria-designed DehydraTECH enhancements (“Enhanced DehydraTECH”) comprised of eleven separate animal studies and released initial results during May 2019 demonstrating measurable quantities of cannabidiol into blood in as little as 2 minutes. In the first animal study results it announced, Lexaria compared its standard DehydraTECH formulation that combined cannabinoids with long-chain fatty acids (“LCFA”) using Lexaria’s patented dehydration processing technique to a concentration-matched formulation utilizing coconut oil which is a commonly used MCT oil in the cannabis edibles industry, with some key findings noted:
| · | At 2 minutes DehydraTECH’s LCFA formulation delivered measurable CBD in blood, compared to no measurable CBD in blood until 6 minutes and onwards for the MCT oil formulation. |
|
|
|
| · | At 60 minutes DehydraTECH’s LCFA formulation achieved a CBD blood concentration level of 319% more than the MCT oil formulation. |
|
|
|
| · | Over the entire 60-minute study, the area under the curve (AUC) (total quantity of CBD delivered) for the Lexaria DehydraTECH LCFA formulation was 389% more than the MCT oil formulation (p<0.0011). |
Lexaria also tested for brain tissue concentrations to quantify 8-hour CBD delivery from the DehydraTECH-enabled LCFA formulation compared to the MCT oil formulation and DehydraTECH’s LCFA formulation outperformed the MCT oil formulation by 246%.
The Company released additional results from its March 2019 research program wherein animal testing proved that Enhanced DehydraTECH delivered 1,137% more CBD into animal brain tissue following oral ingestion than certain existing industry formulations. Delivery of CBD into the brain was reported 8 hours after dosing, as follows:
| · | The Lexaria DehydraTECH LCFA formulation without nanotech achieved an average brain tissue accumulation level that was 246% higher than the average for those animals that received the MCT oil formulation (p=0.0013). |
|
|
|
| · | The Lexaria DehydraTECH LCFA formulation with nanotech achieved an average brain tissue accumulation level that was 1,137% higher than the average for those animals that received the MCT oil formulation (p=0.0178). |
Further results demonstrated that Enhanced DehydraTECH led to 811% more CBD delivery into blood than generic industry MCT coconut-oil formulations (p=0.00008); and 110% more CBD into blood than DehydraTECH in its traditional format (p=0.02).

| Page 15 of 90 |
| Table of Contents |
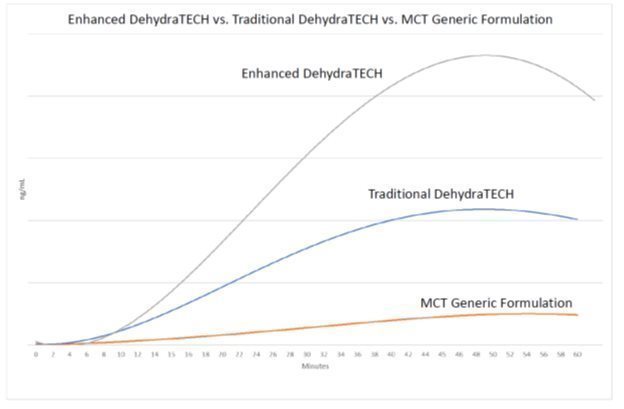
| · | Enhanced DehydraTECH delivered roughly twice as much CBD to animal blood at all measured time points in the study from the 15-minute mark onwards, compared to traditional DehydraTECH; and during the same time points from 717% to 1098% more CBD than the generic industry MCT coconut oil formulations. |
|
|
|
| · | Enhanced DehydraTECH delivered 1,937% more CBD into animal brain tissue after 8 hours compared to generic industry MCT coconut oil formulations; and 487% more than traditional DehydraTECH. Both traditional DehydraTECH and Enhanced DehydraTECH delivered maximum blood concentration levels prior to the 60-minute end-of-test, with levels tapering off thereafter. |
During 2021, we also commenced further DehydraTECH formulation enhancement and performance optimization work. In May of 2021, we announced findings from Study HYPER-A21-1 that included three new "DehydraTECH 2.0" CBD formulation variations. All three new DehydraTECH 2.0 formulations delivered improved performance when compared to both Lexaria's original DehydraTECH and Enhanced-DehydraTECH concentration-matched formulations, as well as to a MCT oil-based control formulation representative of standard industry practices. These three DehydraTECH 2.0 formulations delivered between 1,068% and 2,178% more CBD during the study period than the standard MCT control formulation, and they also were up to 123% more effective than Lexaria’s original Enhanced DehydraTECH formulation. The three new DehydraTECH 2.0 formulations also delivered between 907%-1,737% more CBD into brain tissue than the MCT oil-based control formulation, similar to the up to 1,937% increase over the MCT oil based control formulation determined previously for Lexaria's original Enhanced DehydraTECH formulations.
Also during 2021, further DehydraTECH 2.0 formulation work was reported later in May from Study HYPER-A21-2. One of the DehydraTECH 2.0 formulations tested in this study produced the strongest absorption enhancement results Lexaria has ever recorded, at 2,708% more CBD into bloodstream during the study period than the representative industry standard MCT control formulation.

| Page 16 of 90 |
| Table of Contents |
Based on our many successes in enhancing CBD absorption in animals, and pursuant to our initial success in reducing blood pressure in our 2018 clinical study, Lexaria progressed to more advanced clinical studies later in 2021.
In July of 2021, we reported findings from Study HYPER-H21-1, in which human blood pressure was reduced across both male and female volunteers and was most pronounced with DehydraTECH-CBD in the first 10-50 minutes of the study. Blood pressure reduction from baseline was greatest when measured via systolic pressure. In a subset of volunteers who were Stage 2 hypertensive, peak systolic blood pressure reductions from baseline were observed of as much as approximately 13 mmHg by the 50-minute time point with DehydraTECH-CBD, and systolic BP remained depressed throughout almost the entire 3-hour duration of the study. There was also a tendency for a greater reduction in relative diastolic pressure from baseline with DehydraTECH-CBD than the concentration matched, generic CBD control tested in this study. This was most notable in the initial 10 to20 minute period post-dosing evidencing statistical significance at the 20-minute timepoint (p=0.025). As well, there was a tendency for relative Mean Arterial Pressure ("MAP") to be reduced greater from baseline with the DehydraTECH-CBD than the concentration matched, generic CBD control; again, most notably in the initial 20 minutes post-dosing. By comparison, in Lexaria's 2018 human clinical study, 120 minutes were required to achieve the same level of MAP reduction, demonstrating superior rapidity of onset of the CBD formulation used in Study HYPER-H21-1 relatively speaking. Lexaria was also pleased that its DehydraTECH-CBD was well tolerated by all subjects, with no serious adverse events or side effects observed or reported.
In September of 2021, we reported findings from Study HYPER-H21-2, in which human blood pressure was significantly reduced using DehydraTECH-CBD through the course of a 24 ambulatory study design. At selected times during the 24-hour study, volunteers with mild to moderate hypertension averaged as much as a 20 mmHg (i.e., 23%) decrease in BP relative to placebo. Over the 24-hour ambulatory monitoring period, volunteers averaged a significant reduction of 7.0% (p < 0.001) in systolic pressure, a significant reduction of 5.3% (p < 0.001) in MAP and a significant reduction of 3.5% in diastolic pressure relative to an increase in diastolic pressure (-0.8 vs. +2.7; p<0.001) from baseline with DehydraTECH-CBD relative to placebo. Also, of note, DehydraTECH-CBD triggered its most significant effects upon blood pressure attenuation through the overnight period while subjects slept and in the early morning period. This observation could have tremendous value therapeutically as these periods of the day are most often associated with cardiac stress and infarct events in hypertensive patients when people rise suddenly from and/or become increasingly active relative to the supine/sleeping state.
At the time of this filing, Lexaria has reported that it is pursuing two additional clinical studies that will also investigate the safety and effectiveness of DehydraTECH-CBD for hypertension (i.e., Studies HYPER-H21-3 and HYPER-H21-4). In addition, Lexaria has reported that it has also formally begun the process toward preparation and filing of an Investigational New Drug (“IND”) application with the Food and Drug Administration (“FDA”) with its DehydraTECH-CBD as a prospective registered pharmaceutical treatment for hypertension.
Beyond Lexaria’s hypertension pursuits with DehydraTECH-CBD, it also announced in November of 2021 that it has commenced important new investigational work (Study EPIL-A21-1) exploring whether DehydraTECH-CBD evidences superior ability to inhibit seizure activity compared to both generic CBD and the world’s only licenced pharmaceutical CBD formulation for treating seizure disorders, Epidiolex. And, looking forward to 2022, Lexaria has also announced that it is expecting to conduct additional animal studies to evaluate the potential benefits of DehydraTECH-CBD for other disease conditions of interest, including dementia via Study DEM-A22-1, rheumatoid conditions via Study RHEUM-A22-1 and diabetes via Study DIAB-A22-1.

| Page 17 of 90 |
| Table of Contents |
We have also completed our first study evaluating DehydraTECH used in a topical cream formulation for absorption of CBD through human skin. Results proved significant increases in both speed and quantity of CBD absorption through skin when compared to control formulations. The absorption study was performed on human skin at a California-based laboratory that specializes in Franz diffusion cell skin permeability testing. DehydraTECH was used together with a sophisticated oil-in-water emulsion formulation design and compared to a series of matching oil-in-water emulsion formulations prepared with the same CBD inputs, with and without DehydraTECH and with and without two leading skin penetration enhancers currently used in the skin products industry. Several factors were measured, including the time required to detect CBD skin penetration and quantity, and peak amounts of CBD absorbed into and through the skin, at multiple testing intervals over a 48-hour duration.
Lexaria’s DehydraTECH-enabled topical formulation, absent either of the commercial penetration enhancers, was the fastest acting for absorption into the epidermis, dermis or through the skin into the systemic fraction representing permeation into the underlying circulatory system.
Furthermore, Lexaria’s DehydraTECH-enabled topical formulation without the addition of either of the commercial penetration enhancers, demonstrated the highest overall average quantity of CBD delivered through the skin and into the representative systemic fraction of all the formulations tested, with as much as a 225% increase in CBD permeability when compared to the highest performing commercial penetration enhancer formulation assessed and almost a 1,900% increase in CBD permeability when compared to a control formulation that was devoid of both DehydraTECH or any commercial penetration enhancers. The commercial skin penetration enhancers only demonstrated performance that was on par or superior to the DehydraTECH-enabled formulations tested in so far as total CBD absorption into the shallow epidermis or dermis was concerned.
Finally, beyond CBD, we also conducted investigative work with another cannabinoid compound, THC, during 2021. In Study THC-A21-1, we demonstrated that DehydraTECH-THC delivered via oral ingestion required only 15 minutes to deliver THC levels in blood plasma comparable to levels achieved at 45 minutes with concentration-matched controls. During the study DehydraTECH-THC delivered more THC into bloodstream than the industry standard MCT based control formulation from the 2-minute mark onwards, then dropped rapidly to the same level as the MCT control by the 6-hour mark. These data may be of significance to prospective pharmaceutical applications for DehydraTECH-THC based therapeutics, pending further pursuit in this area.
Nicotine Programs
We have also completed ingestible nicotine in vivo (animal) absorption study work. In a study reported in April of 2018, DehydraTECH delivered the following major nicotine absorption performance improvements: 1,160% faster delivery of equivalent peak quantities of nicotine to the bloodstream than achieved with controls (within 15 min vs. 2.9 hours), 148% gain in the quantity of peak nicotine delivery to the bloodstream relative to controls, 560% higher brain levels of nicotine where nicotine effects are focused, compared to controls, lower urine levels of nicotine excreted than controls, for enhanced nicotine activity and bioavailability over the course of the study, lower quantities of key liver metabolites in the bloodstream than controls as hypothesized, suggesting bypass of first pass liver metabolism.
The study was designed to principally assess the relative ingestible nicotine absorption performance of DehydraTECH -powered formulations compared to concentration-matched control formulations that lacked any form of delivery enabling technology in rats.
The DehydraTECH formulations generally achieved faster absorption, higher peak absorption, and higher overall quantities of nicotine, on average, in the blood than the concentration-matched control formulations at both the 1mg and 10 mg/Kg doses tested. Furthermore, as previously reported, there were no obvious signs of gastrointestinal distress such as vomiting or diarrhea indicating that the animals appeared to tolerate the treatment well.

| Page 18 of 90 |
| Table of Contents |
Nicotine blood levels were evaluated multiple times over a period of 8 hours after dosing. In the 10mg/Kg dosing arm, the control formulation required nearly 3 hours to reach similar levels of blood absorption that the DehydraTECH formulation reached in only 15 minutes. Furthermore, the DehydraTECH formulation went on thereafter to demonstrate peak plasma levels that were 148% of those achieved by the control formulation. If replicated in human studies, these findings are suggestive that DehydraTECH could prove more effective in elevating blood nicotine levels through edible formats much more quickly and substantially than previously theorized, potentially making ingestible nicotine preparations a viable alternative to today’s available product formats while also leading to a more rapid nicotine craving satiation.
Analysis of the liver metabolites revealed, as expected, that overall levels in the blood of two of the three metabolites studied were higher in the control group than in the DehydraTECH formulation group at the 10 mg/Kg dose. The study also revealed that the DehydraTECH formulation at the 10 mg/Kg level achieved up to 5.6-times as much nicotine upon analysis of the rat brain tissue than was recovered with the matching control formulation. These findings together perhaps suggest prolongation of nicotine effectiveness with the DehydraTECH formulation which may also be beneficial in humans to control cravings over an extended time-period from a single edible nicotine dose.
Following the above study, additional study work was reported in August of 2018 by way of a follow-up third-party in vivo statistically significant study, including two groups of 20 animals. This study further demonstrated delivery of nicotine in edible form at each of the 2, 4, 6, 8 and 10-minute intervals post-dosing, with 90.2% greater delivery than the concentration-matched control formulation by the 10-minute mark (95% CI; p=0.044), and significantly greater absorption levels than the control formulation at all subsequent time points in the study. Speed of onset is a key attribute for oral drug administration, and it is of particular importance for the consideration of non-inhalation nicotine delivery formats.
Key highlights of the follow-up study were as follows:
| · | Peak Level: 79% improvement in peak blood levels (maximum concentration or “Cmax”) at 394 ng/mL using Lexaria’s DehydraTECH technology vs. 220 ng/mL with the control (95% CI; p=0.0257); |
|
|
|
| · | Total Quantity: 94% improvement in total quantity of nicotine delivered (area under the curve or “AUC”) to the blood during the 60-minute course of the study, at 266 hr•ng/mL versus 137 hr•ng/mL (95% CI; p=0.0086); |
|
|
|
| · | Rapidity: Lexaria’s technology delivered nicotine into the blood stream by the first time interval of blood sampling at the 2-minute mark. On average, Lexaria’s technology delivered 203 ng/mL to the blood in aggregate of the 2, 4, 6, 8, 10, 12 and 15-minute time points, compared to only 120 ng/mL in aggregate over the same period by the control, an improvement of 70% (95% CI; p=0.0004). |
Thereafter, during 2021, we also pursued study work in animals to investigate the pharmacokinetic performance of certain DehydraTECH 2.0 nicotine formulations specifically via the oral buccal/sublingual route of administration instead of the oral ingestible route of administration investigated previously. In October of 2021, we reported upon Study NIC-A21-1 conducted in male beagle dogs, which demonstrated that DehydraTECH nicotine delivered via the oral pouch product format required only 2 to 4 minutes to deliver nicotine levels in blood plasma comparable to levels achieved at 45 minutes with concentration-matched controls. DehydraTECH-nicotine also reached statistically significant peak blood plasma levels up to 10-fold higher overall than controls (p=0.004) while still clearing from blood virtually as quickly as the controls. Two nicotine formats were investigated in Study NIC-A21-1, namely nicotine benzoate and nicotine polacrilex. In the study, the generic nicotine benzoate pouch required approximately 45 minutes to reach its peak delivery rate whereas the DehydraTECH nicotine benzoate pouch reached peak delivery rates at both 8 minutes and again at 30 minutes. In fact, just 4 minutes after the pouch was placed in the mouth, the DehydraTECH-nicotine had reached a higher delivery level than the generic achieved at any point during the study. Similarly, the generic nicotine polacrilex pouch also required approximately 45 minutes to reach its very subdued peak delivery rate while the DehydraTECH nicotine polacrilex pouch achieved a comparable level in just 2 minutes. The DehydraTECH nicotine polacrilex pouch delivered over 10 times the nicotine level in blood plasma at peak than the generic version.

| Page 19 of 90 |
| Table of Contents |
Antiviral Drug Programs
During March of 2020, we also announced that we were commencing a program to study the prospective benefits of Lexaria’s DehydraTECH drug delivery platform for enhancing delivery and effectiveness of certain antiviral drugs in the fight against coronavirus disease COVID-19. We commenced this work initially reporting upon improved delivery in an animal study conducted with the antiviral drugs darunavir and efavirenz, with which we reported significant enhancements in drug delivery announced in December of 2020 in Study VIRAL-A20-1. Thereafter, we progressed to further study work with other drugs with antiviral properties including remdesivir and ebastine, where we demonstrated improved drug delivery in animal testing in Study VIRAL-A20-2, as well as effective inhibition of the SARS-CoV-2 virus that is responsible for COVID-19 in in vitro testing via Study VIRAL-C21-3 reported in June of 2021. Finally, we also reported significant improvements in drug delivery in animal using another compound with antiviral properties, colchicine, in Study VIRAL-A20-3, announced in July of 2021.
Technology out-licensing
Pursuant to the disposition of assets of CanPharm, all of the Company’s licenses associated with THC molecules for non-pharmaceutical purposes were assigned to Hill Street. As part of an asset purchase agreement entered into between CanPharm and Hill Street, on November 18, 2020, Lexaria Hemp Corp. (“Hempco”) entered into a 10-year license agreement with Hill Street to license DehydraTECH with respect to multiple products infused with CBD, replacing all previous agreements between the parties.
On January 15, 2019, the Company announced that its wholly owned subsidiary Lexaria Nicotine granted a license to use the DehydraTECH for oral nicotine delivery forms on an exclusive basis in the United States and a non-exclusive global basis to Altria Ventures Inc., an indirect wholly owned subsidiary of Altria Group, Inc. (“Altria”). During fiscal 2021, Altria relinquished their exclusive rights and retain non-exclusive rights within the U.S.
On July 11, 2019, the Company announced that it entered a definitive 5-year agreement, via its subsidiary Hempco, to license DehydraTECH to Universal Hemp LLC, for the production of infused powder substrates for inclusion in finished goods. This license was subsequently terminated on August 30, 2021.
On December 27, 2019, the Company, via Hempco entered into a 10-year US exclusive license agreement with Boldt Runners Corporation (“Boldt Runners”) for the use of DehydraTECH in connection with manufacturing CBD infused oral pouch products. Subsequent to the year ended August 31, 2021, Boldt Runners relinquished their exclusive rights and maintain non-exclusive rights.
Subsequent to the year end, on September 16, 2021, Hempco entered into a 10-year license with GlobalCanna Inc. for the use of DehydraTECH in multiple CBD infused products in the country of Canada.

| Page 20 of 90 |
| Table of Contents |
The continuation of our business interests in these sectors is dependent upon obtaining further financing, a successful program of development, and, ultimately, achieving a profitable level of operations. The issuance of additional equity securities by us could result in a significant dilution in the equity interests of our current stockholders. Obtaining commercial loans, assuming those loans would be available, will increase our liabilities and future cash commitments.
We are not yet profitable and have not yet demonstrated our ability to generate significant revenues from our business plan. We will require additional corporate funds if our existing capital is not sufficient to support the Company until potential future profitability is reached. There are no assurances that we will be able to obtain further funds required for our long-term operations. We expect to require additional operating capital during our fiscal 2021 year. There can be no assurance that additional financing will be available to us when needed or, if available, that it can be obtained on commercially reasonable terms. If we are not able to obtain the additional financing on a timely basis, we will be unable to conduct our operations as planned, and we will not be able to meet our other longer-term obligations as they become due. In such event, we could be forced to scale down or perhaps even cease our operations. There is uncertainty as to whether we can obtain additional long-term financing if we do in fact require it.
We hired two additional staff members during fiscal 2021 to enhance operations in our office and licenced laboratory space. We currently have eight staff members and do not anticipate hiring large numbers of new staff members during fiscal 2022. We expect to be able to utilize contracted third parties for our R&D testing programs, instead focusing our capital on higher value-added aspects of our research and development, and scientific test planning.
Our Company relies on the business experience of our existing management, on the technical abilities of consulting experts, and on the technical and operational abilities of its operating partner companies to evaluate business opportunities.
Competition
The biopharmaceutical industry is characterized by intense competition and rapid innovation. Our competitors may be able to develop other drug delivery platforms that are able to achieve similar or better results than DehydraTECH. Our potential competitors include major multinational pharmaceutical companies, established biotechnology companies, specialty pharmaceutical companies, universities, and other research institutions. Many of our competitors have substantially greater financial, technical, and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations and well-established sales forces. Smaller or early-stage companies may also prove to be significant competitors, particularly as they develop novel approaches to oral or topical drug delivery that our DehydraTECH is also focused on. Established pharmaceutical companies may also invest heavily to accelerate discovery and development of novel therapeutics or to in-license novel therapeutics that could make the product candidates that are can be delivered using DehydraTECH obsolete. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors, either alone or with collaborative partners, may succeed in developing, acquiring, or licensing API delivery technologies that are more effective, safer, more easily commercialized or less costly than our DehydraTECH proprietary technology or secure patent protection that we may need for the enhancement of our DehydraTECH. We believe the key competitive factors that will affect the development and commercial success of any DehydraTECH enhanced product candidates are efficacy, safety, tolerability, reliability, convenience of use, price, and reimbursement. We face competition from segments of the pharmaceutical, biotechnology and other related markets that pursue the development of API delivery platforms which may be more effective or cost efficient than our DehydraTECH. We anticipate that we will continue to face intense and increasing competition as new advanced API delivery technologies become available. There can be no assurance that our competitors are not currently developing, or will not in the future develop, technology that is equally or more effective or is more economically attractive than any of our current or any enhanced versions of DehydraTECH.

| Page 21 of 90 |
| Table of Contents |
Competition in alternative health sectors and in consumer products in the U.S. is fierce. We expect to encounter competitive threats from existing participants in the sector and new entrants with competing technologies. Although PoViva Corp. has filed patent applications to protect intellectual property, there is no assurance that patents beyond those already issued will be granted nor that other firms may not file superior patents pending. Food supplements, organic foods, and health food markets are all well established and the Company and/or its licensees will face many challenges within these markets. Lexaria is also aware of various competing technologies that exist in the marketplace that claim to also enhance the bio absorption of bioactive molecules as Lexaria has demonstrated through repeated in vitro and in vivo scientific testing with DehydraTECH. By and large, these technologies are mostly forms of nanotechnology that generally claim to enable the formation of microencapsulated microemulsions of active ingredients. These technologies can enable exceptional water solubility of ingredients and can impart improved intestinal bio absorption as a result, but do not necessarily offer the breadth of performance and value enhancing benefits that Lexaria’s DehydraTECH technology offers to its licensees.
Competition in nicotine, alternative nicotine delivery and nicotine cessation sectors in the U.S. is comprised of long-established entities, brands, and new technologies competing to create less harmful options. The sectors are complicated by the significant historical empirical data of older products or technologies versus the more limited published supporting data regarding the effects of new products or technologies. Due to the size of the sectors we expect to encounter competitive threats from existing participants and unknown new entrants. There is no assurance that other technologies already deployed, or in development, will not form the basis of product formats that competitors or consumers choose to utilize. It is also possible that historic delivery methods that have been in use and the familiarity with them may prevent adoption of products utilizing DehydraTECH in alternative delivery formats. Competing technologies or products may utilize known delivery formats or entirely new and unforecastable formats. Lexaria has demonstrated through scientific testing that DehydraTECH delivers nicotine rapidly and effectively through oral delivery. We believe that if we can educate and influence consumers to adopt a food-grade edible product format, and if US regulatory bodies authorize such formats, we may be able to offer a competitively successful new product format that utilize DehydraTECH.
While we are an early adopter providing technology to the cannabinoid sector, there are reports of large numbers of public companies that have claimed to be involved in the sector in some fashion, and an unknown number of private companies. Our current strategies may prove to be ineffective as the sector grows and matures, and if so, we will have to adapt quickly to changing sectoral circumstances. Accordingly, the Company intends to aggressively pursue technology out-licensing opportunities not only within the cannabinoids sector where it is already active, but also across other sectors where DehydraTECH is patent allowed and/or pending, include opportunities in the vitamin and supplements sector, the pain relief sector, and the nicotine products sector. During the year ended August 31, 2021 the Company sold the rights to DehydraTECH using THC and no longer involved in this market segment.

| Page 22 of 90 |
| Table of Contents |
Lexaria believes that DehydraTECH offers a host of benefits beyond what competing technologies can offer, including superior oral palatability, a more appealing and all-natural ingredient compositional profile from an oral product and beverage formulation perspective, more predictable time of delivery into bloodstream and certain target tissues, and superior scalability and cost effectiveness from a manufacturing perspective. Lexaria believes that DehydraTECH is, therefore, significantly distinguished from competing technologies in these respects, and has a view of growing the breadth and number of licensees that will adopt DehydraTECH into their product offerings going forward. Lexaria believes that these competitive advantages together with its wealth of scientific data showing noteworthy bio absorption enhancements with DehydraTECH constitute a compelling value proposition for its prospective licensees, and it intends to continue to pursue license arrangements in the multiple bioactive ingredient sectors identified in its issued and pending patent applications.
Compliance with Government Regulation
Thirty-nine states in the U.S. have passed some form of legislation related to that state’s permission to grow, cultivate, sell, or use marijuana and/or CBD either for medical purposes or for recreational or “adult use” purposes, or both (disa.com). The various state legislation is not necessarily harmonious with one another. It is most often not legal to transport cannabis-related products across state lines.
Lexaria does not “touch the plant” or culture, manufacture, process, handle or sell cannabis in any location within the U.S. Lexaria does conduct research and development on cannabis ingredients legally in Canada, in a federally licensed laboratory in compliance with all federal and local Canadian laws. We comply with U.S. federal law that provides for certain exemptions for agricultural hemp and certain by-products to be manufactured and sold in the U.S. DehydraTECH is only licensed to those companies that have met and comply with state regulations for the sale or distribution of cannabis related products in the licensed territory where they operate. Lexaria does not currently manufacture or sell any CBD or THC-related consumer products.
Lexaria’s position is that, just as a telephone company provides communications services, and an electric company provides electrical power, our provision of technological services to a state-legal cannabis company is in compliance with laws and required regulations. Lexaria disposed of its assets that were related to the provision of technology to THC-related companies in December of 2020.
DehydraTECH also has applications in completely separate sectors such as vitamins, CBD for applications under pursuit for medical applications registered with the FDA, NSAIDs, and nicotine. We have no products nor operations in any of these sectors today, although we have commenced formulation development for research and validation purposes in each of these areas. We have a formal relationship with the largest cigarette company in the U.S., the Altria Group, and have conducted R&D with that company related to the possible development of nicotine oral products. We do not know whether the Altria Group will utilize DehydraTECH within any oral nicotine product category. If we enter any of these sectors at any time, we will be exposed to and of necessity will have to comply with, all local, state, and federal regulations in each of those sectors. As a result of the possibility of Lexaria being involved in a number of disparate business sectors, compliance with government regulations could require significant resources and expertise from our Company.
The U.S. Farm Bill, which passed in December 2018, and the ambiguity regarding the incorporation of CBD into ingested and topical products has had significant impacts on the industry segments that we operate and potentially changes some of the regulatory compliance risks that may affect our business. The bill includes lifting restrictions on advertising, marketing, banking, and other financial services as well as allowing interstate commerce for hemp and hemp-derived CBD, removing barriers for intellectual property protections under federal law such as patents and trademarks, as well as several other measures that may positively impact these industry segments overall. The impact the Bill may have on other regulatory bodies and their regulations will require ongoing monitoring to determine the outcome and timing of any revisions.

| Page 23 of 90 |
| Table of Contents |
Employees and Contractors
We utilize employees, sub-contractors and consultants for the company’s intellectual property development and licensing, and business operations. We have six employees and may add research personnel during the next 12-month period to expand our internal R&D capacity. None of our employees is represented by a labor union and we consider our employee relations to be good. We also engage with consultants to serve our needs.
The Company has an agreement with CAB Financial Services Ltd., wholly owned by Chris Bunka, for a 3-year term management contract as Chief Executive Officer effective January 1, 2019. The Company, as of the date of this Form-10K, is negotiating a contract agreement with Mr. Bunka to take effect January 1, 2022.
The Company has engaged Mr. John Docherty for a 3-year term effective January 1, 2019 as its President. The Company, as of the date of this Form-10K, is negotiating a contract agreement with Mr. Docherty to take effect January 1, 2022.
Both of the Chief Executive Officer and the President of the Company are entitled to the following performance incentives:
| · | a performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the board of directors of Lexaria; |
|
|
|
| · | compensation equal to 2% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances; |
|
|
|
| · | certain compensation to be paid upon a change of control excluding certain circumstances; and |
|
|
|
| · | participation in the Company’s approved equity incentive plan. |
Our business plan contemplates increases in the number of employees and other personnel over the next 12-month period to enhance operational, sales and our in-house R&D capacity dependent upon adequate funding. When beneficial to do so we will continue to outsource contract employment or engagements as needed. It is not possible to accurately project potential needs into the future based on circumstances that may or may not occur.
Research and Development
Lexaria incurred $1,262,895 (2020 - $387,074) in research and development expenditures during the period ending August 31, 2021. Specific R&D programs are in ongoing development and will be tightly related to our financial ability to undertake each research phase for each API. Due to our expanding portfolio coverage, we are continuing to examine accelerated timetable options for testing, research and development.
The Company’s in vitro absorption test of DehydraTECH enhanced nicotine molecules and its in vivo absorption tests on DehydraTECH enhanced CBD molecules yielded positive results. Ongoing testing plans are proceeding to (i) conduct in vitro absorption tests with DehydraTECH enhanced ibuprofen; and (ii) further define molecular compatibility, absorption rates, timing, and viable formats of delivery.

| Page 24 of 90 |
| Table of Contents |
The Company continually focuses on new R&D programs to investigate potential additional commercial applications for the incorporation of DehydraTECH. These include, but are not limited to, ongoing programs to explore methods to integrate nanoemulsification chemistry techniques together with DehydraTECH that have demonstrated positive results to date, programs to further enhance intestinal bio absorption rates with DehydraTECH, as well as ongoing programs to expand the types and breadth of product form factors into which DehydraTECH can be applied. Depending on how many of these tests are undertaken, R&D budgets are expected to vary significantly. It is in our best interests to remain flexible at this early stage of our R&D efforts in order to capitalize on potential novel findings from early-stage tests and thus re-direct research into specific avenues that offer the most reward.
Subsidiaries
Lexaria has its wholly owned subsidiaries; Lexaria CanPharm ULC, Lexaria CanPharm Holding Corp., PoViva Corp., Lexaria Hemp Corp., Kelowna Management Services Corp. and Lexaria Pharmaceutical Corp., and our majority owned subsidiary Lexaria Nicotine LLC. On January 15, 2019, the Company announced the initial investment of $1,000,000 from Altria Ventures Inc., an indirect wholly owned subsidiary of Altria Group, Inc., for a 16.667% equity interest along with certain other rights in Lexaria Nicotine LLC.
Item 1A. Risk Factors
Summary of Risk Factors
The following table summarizes the material risk factors associated with our Company which are more fully described below:
A. | Risks Associated with our Business and Industry | |
| (i) | Business operations |
| (ii) | Protection of intellectual property and litigation |
| (iii) | Reliance on third party providers |
| (iv) | COVID-19 |
|
|
|
B. | Risks Associated with our Financial Condition | |
| (i) | Historical net losses and reliance on licensing |
| (ii) | Additional funding requirements for R&D activities |
| (iii) | Additional Funding Requirements for Business Plan |
|
|
|
C. | Risks Associated with Current Regulatory Environments | |
| (i) | Conducting clinical trials |
| (ii) | Regulatory and development approvals for pharmaceutical products |
| (iii) | Controlled substances |
|
|
|
D. | Risks Associated with Securities Markets and Ownership of our Common Stock | |
| (i) | Pricing volatility of common stock and warrants |
| (ii) | Strategic transactions |
| (iii) | Payment of dividends and dilution |
| (iv) | Smaller reporting company compliance |
|
|
|
E. | General Risks | |

| Page 25 of 90 |
| Table of Contents |
A. Risks Associated with our Business and Industry
(i) Risks related to our business operations
We face substantial competition, which may result in others discovering, developing and/or commercializing technology or products similar to ours before or more successfully than we might do.
Lexaria operates in the intensely competitive biotechnology industry. Investment in this sector involves a high degree of risk.
Our commercial and/or licensing opportunities may be reduced or potentially eliminated if our competitors develop and commercialize products utilizing a similar technology that compete directly with those incorporating DehydraTECH. Significant delays in the development of our product candidates could allow competitors to bring products to market before us which may impair the ability to commercialize our product candidates. This could result in reduced sales and increased pricing pressure on our technology which in turn would reduce our ability to generate meaningful revenues and could have a negative impact on our results of operations.
Our competitors might also develop drugs that are more effective, more widely used and less expensive than ours, and they may also be more successful in manufacturing and marketing their products. Competitors could acquire regulatory approval of their products before we are able to obtain patent protection or other intellectual property rights, limiting our ability to license our respective patents and/or develop or commercialize a product candidate. These appreciable advantages could render our product candidates non-competitive or obsolete before we can recover the expenses of research, development, and commercialization.
Our competition includes pharmaceutical and biotechnology companies, educational institutions, and research foundations, many of which have substantially greater capital resources, research and development staffs and facilities and greater marketing experience than Lexaria. They may be able to respond more rapidly to new regulations and/or devote greater resources to the development and promotion of their business model. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or potentially advantageous to our business.
Early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors and could increase their ability to rapidly gain market share.
As a result of these factors, management cannot be certain that the Company will be able to compete against current or future competitors or that competitive pressure will not seriously harm its business.

| Page 26 of 90 |
| Table of Contents |
Our failure to protect our intellectual property may have a material adverse effect on our ability to develop and licence DehydraTECH
Because patents involve complex legal and factual questions, the issuance, scope, validity, and enforceability of patents cannot be predicted with certainty. Some of our patent pending applications may not be granted as patents. Even if patents are issued, they may not be issued with claims of sufficient breadth to protect DehydraTECH technology or may not provide us with competitive advantage against competitors with similar products or technologies. Issued patents may be challenged, invalidated, or circumvented. If patents issued to us are invalidated or found to be unenforceable, we could lose the ability to exclude others from making, using, or selling the inventions claimed. Moreover, an issued patent does not give us the automatic right to use the patented technology or commercialize a product using the technology. Third parties may have blocking patents that could be used to prevent us from developing our products, selling our products, or commercializing our DehydraTECH technology. Others may also independently develop products or technologies similar to those that we have developed or may reverse engineer or discover our trade secrets through proper means.
Results of earlier studies may not be predictive of future results and planned or ongoing studies may not establish an adequate efficacy profile for DehydraTECH-enabled products.
The results of studies and trials of DehydraTECH conducted to date and future studies incorporating other APIs may not be predictive of the results of subsequent trials. Studies published to date on DehydraTECH have demonstrated positive results through oral and topical delivery methods of API payloads. These results may not be replicated in subsequent studies or trials that incorporate the same or other API payloads.
Licensees subject to significant regulatory requirements and testing protocols, such as those required by the US Food and Drug Administration (FDA), and comparable foreign regulators, must successfully complete multi-phase testing and the results of our studies may not be reflected in the outcome of the testing performed related to their products. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier studies, and we cannot be certain that our licensees will not face similar setbacks.

| Page 27 of 90 |
| Table of Contents |
Intellectual Property and Technology development involves a lengthy and expensive process, with an uncertain outcome. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, all of the research and development for all industry segments.
We may experience delays in initiating or completing our planned studies or trials in the future, and we may experience numerous unforeseen events during, or as a result of, any future studies or trials that we conduct that could delay or prevent our ability to conduct the research, including:
| · | regulators or institutional review boards (“IRBs”), or ethics committees may not authorize us or our investigators to commence a study or trial at a prospective trial site and/or additional governmental regulatory authority authorizations may be required from time-to-time to do so for which there is no assurance that we will be able to satisfy their approval conditions in a timely fashion if at all, whether due to financial or other unforeseen constraints; |
| · | we may experience delays in reaching, or fail to reach, agreement on acceptable terms with prospective trial sites and prospective contract research organizations (“CROs”), the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| · | we may experience delays in recruiting, or be unable to recruit, a sufficient number of suitable participants to participate in our studies or trials; |
| · | the participants and sites who participate in our studies or trials may not comply with required protocols rendering the results insufficient or uninterpretable; |
| · | studies or trials of various APIs may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional studies or trials or we may decide to abandon development programs related to those APIs; |
| · | the number of participants required for studies or trials of an API may be larger than we anticipate, enrollment in these studies or trials may be slower than we anticipate or participants may drop out or fail to return for follow-up at a higher rate than we anticipate; |
| · | our third-party contractors may fail to comply with regulatory or legal requirements or meet their contractual obligations to us in a timely manner, or at all, or may deviate from the protocol or drop out, which may require that we find new contractors to perform the work; |
| · | we may elect to, or regulators or IRBs or ethics committees may require that we or our investigators, suspend or terminate our research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risk; |
| · | the cost of studies or trials of an API may be greater than we anticipate; |
| · | any changes in regulatory requirements and guidance that require amending or submitting new protocols; |
| · | regulators may require us to submit additional data or impose other requirements before permitting us to initiate a study or trial. |
We could encounter delays if a study or trial is suspended or terminated by us or by the IRBs of the institutions in which they are being conducted. Such authorities may impose such a suspension or termination due to a number of factors, including changes in governmental regulations or administrative actions or lack of adequate funding to continue the study or trial. Further, the IRB may disagree with our design or may change the requirements for approval even after it has reviewed and commented on the design.
Our research and development costs will also increase if we experience delays in testing or regulatory approvals. We do not know whether any of our studies or trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Any delays in our development programs may significantly harm our business, prospects, financial condition, and results of operations.

| Page 28 of 90 |
| Table of Contents |
(ii) Risks related to protection of intellectual property and litigation
If we are unable to obtain and maintain sufficient patent protection, or if the scope of the patent protection is not sufficiently broad, our competitors could develop technology similar to ours.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our intellectual property. If we do not adequately protect or enforce our intellectual property, competitors may be able to erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. To protect our intellectual property, we file patent applications in the United States and abroad. The patent application and approval process is expensive, complex and time-consuming. We may not be able to effectively enforce our intellectual property rights throughout the world. Filing, prosecuting, and defending patents in all countries throughout the world would be prohibitively expensive. Our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, the patent laws of some foreign countries do not provide protection to the same extent as the laws of the United States. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our intellectual property rights. Legal actions to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and resources from other aspects of our business. While we intend to protect our intellectual property, we cannot ensure that we will be able to initiate or maintain legal efforts in all jurisdictions.
(iii) Risks related to our reliance on third party providers
We have relied, and will rely in the future, on third parties to conduct, supervise, and monitor our R&D programs. If third party performance is unsatisfactory, including failing to meet deadlines for the completion of contracts or failing to comply with regulatory requirements our research programs may be delayed or could fail to develop required data.
We do not have the ability to conduct our studies or pre-clinical trials independently and thus rely on third parties to conduct, supervise, and monitor our R&D programs. While we have, or expect to have, agreements governing the activities of such third parties, we will have limited influence and control over their actual performance and activities. Third-party service providers are not our employees, and except for remedies available to us under contract with such third parties, we cannot control whether or not they devote sufficient time, skill and resources to our programs. We remain responsible for ensuring that each of our programs are conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards, and our reliance on third parties will not relieve us of our regulatory responsibilities. We remain responsible for ensuring that each of our trials is conducted in accordance with the general investigational plan and protocols for that trial.
If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our R&D programs or preclinical studies in accordance with our stated protocols or regulatory requirements, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our protocols, regulatory requirements or for other reasons we or other third-party collaborators may be subject to regulatory enforcement or other legal actions. Resultant data generated in our preclinical programs may be deemed unreliable and our studies and trials may need to be repeated, extended, delayed, or terminated. We may be delayed in or unable to obtain marketing approvals for our product candidates or to successfully commercialize our product candidates. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
Agreements with third parties conducting or otherwise assisting with our R&D might terminate for a variety of reasons, including a failure to perform by the third parties. If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative providers or to do so on commercially reasonable terms. Switching or adding additional third parties involve increased management time and focus and additional cost. With a transition to a new third party and alternative arrangements there will be delays in our research programs and this will adversely affect our business. We intend to manage our relationships with third parties carefully and respectfully but there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects, and results of operations.

| Page 29 of 90 |
| Table of Contents |
We rely upon third parties for the manufacture of our B2B products. If those third parties do not perform satisfactorily, including failing to meet deadlines for the completion of such contract or failing to manufacture goods to the exact specifications of our customers, it could lead to our B2B customers dissatisfaction and could harm our reputation and cause loss of revenues.
We rely and expect that we will rely on third party suppliers and manufacturers to provide us with the materials and services to manufacture our DehydraTECH compounds for our B2B customers. While we do have in-house expertise and capacity to manufacture using DehydraTECH, we do not own or lease manufacturing facilities. To the extent we are unable to successfully manage the performance of third-party service providers, our business may be adversely affected. If these third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, or if the quality or accuracy of the product they produce is compromised due to the failure to adhere to our protocols, regulatory requirements or for other reasons, our relationship with our B2B customers may be critically affected and may result in the loss of revenue. Demand for our services may be adversely affected if consumers lose confidence in the quality of our services or the industry’s practices. Adverse publicity may discourage businesses from contracting our services and could have a material adverse effect on our financial condition and results of operations.
The FDA, or equivalent regulatory authority, governs the manufacturing process for product candidates in pre-clinical and clinical trials and will inspect the facilities at which the product is manufactured. Approval of the product will not occur unless the manufacturing facilities are in compliance with the FDA’s current good manufacturing practice (“cGMP”) regulations, or equivalent foreign authority. If our suppliers or manufacturers do not comply with the FDA or foreign regulations for our product candidates, we may experience delays in timing or supply, be forced to manufacture our product candidates ourselves or seek to enter contract with another supplier or manufacturer. If we are required to switch suppliers or manufacturers, we will be required to verify that the new supplier or manufacturer maintains facilities and processes in line with cGMP regulations, which may result in delays, additional expenses, and may have a material adverse effect on our ability to complete the development of our product candidates.
(iv) Risks related to the effects of COVID-19
The outbreak of the coronavirus (COVID-19) has evolved into a global pandemic. The extent to which the virus impacts our business and operating results will depend on future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning the virus, its variants, and the actions to contain the coronavirus or treat its impact, among others.
With the continued spread of the virus, our business operations could be interrupted or delayed. It is possible that our R&D programs could be adversely affected by the pandemic. In some of our programs, particularly our human studies, participant recruitment and enrollment, participant dosing, distribution of results, study monitoring and data analysis may be paused or delayed due to the effects that the pandemic has in different countries, regions, states, provinces, or localities. If the virus continues to spread, some participants and clinical investigators may not be able to comply with clinical trial protocols. For example, travel restrictions, lock-down quarantines or other limitations that might limit our ability to conduct our R&D programs. We currently utilize third parties to conduct our R&D programs and to produce products for our B2B customers. These relationships could be adversely impacted by restrictions resulting from the virus outbreak. It is possible that our supply chain may be disrupted, limiting our ability to manufacture products for our R&D operations or for our B2B customers.
The spread of COVID-19 and its variants, has caused a broad impact globally, including restrictions on travel and quarantine policies put into place by businesses and governments, and it may have a material economic effect on our business. While the potential economic impact brought by and the duration of the pandemic may be difficult to assess or predict, it has already caused, and is likely to result in further significant disruption of global financial markets, which may reduce our ability to access capital either on favorable terms or at all. In addition, inflation, stagflation, recession or other sustained adverse economic events resulting from the spread of the virus could materially and adversely affect our business and the market for or value of our common stock.

| Page 30 of 90 |
| Table of Contents |
B. Risks Associated with our Financial Condition
(i) Risks related to historical net losses and reliance on licensing
Our Company has little operating history and an evolving business model, which raises doubt about our ability to achieve profitability or obtain financing.
Our Company has no significant history of operations and our business model is still evolving and is subject to change. Our revenues are dependent upon licensing DehydraTECH and on those licensees generating usages fees by successfully selling products utilizing DehydraTECH. Without increased market acceptance of our technologies, we may not generate meaningful revenue. Our licensees may also be subject to regulatory approval of their products that utilize DehydraTECH, which may not occur before they can bring their products to market and we generate usage licensing revenues from them.
Our Company’s ability to continue as a going concern is dependent upon our ability to obtain adequate financing for our research and development and our operational requirements and/or to reach profitable levels of operations. In that regard we have no proven history of performance, earnings, or success. Our revenues are primarily generated from out-licensing of DehydraTECH technology. There can be no assurance that we will achieve significant revenues or profitable operations or will generate adequate funds to continue our intellectual property development. Many factors, such as competition, patent protection, appropriate regulatory approvals, availability of personnel, and market acceptance of our services can influence the revenue and profitability potential. As a result, we may experience material fluctuations in future operating results on a quarterly and annual basis which could materially affect our business, financial condition, and operating results. Although we exercise due consideration in the development of our technology, we cannot be certain that our overall business model within any particular sector will ever come to fruition, and if they do, will not decline over time. We may not recover all or any portion of our capital investment in our research and technology development, marketing, or other aspects of the business.
(ii) Risks related to additional funding requirements for R&D activities
The longer-term growth of our business depends on our ability to expand our portfolio of patents and industry segments where DehydraTECH is demonstrably applicable, which may require substantial financial resources and may ultimately be unsuccessful.
The longer-term growth of our business depends upon our ability to expand our patent portfolio of applicable APIs and molecules and delivery methods. We may also be required to evidence that DehydraTECH’s demonstrated efficacy also works with other APIs and molecules prior to acceptance and adoption within those segments. The R&D programs required to develop the evidence may require substantial financial resources and may ultimately be unsuccessful.
(iii) Risks related to additional funding requirements for business plan
Without additional financing to develop our business plan, our business may fail.
Because we have generated only minimal revenue from our business and cannot anticipate when we will be able to generate meaningful revenue from our business, we will need to raise additional funds to conduct and grow our business. We anticipate that we will need to raise further financing. We do not currently have any arrangements for financing and we can provide no assurance to investors that we will be able to find such financing if required. The most likely source of future funds presently available to us is through the sale of equity capital. Any sale of share capital will result in dilution to existing security-holders.

| Page 31 of 90 |
| Table of Contents |
C. Risk Associated with Current Regulatory Environments
(i) Risks related to conducting clinical trials
Our product candidates are in an early stage of development and may fail or experience significant delays or may never advance to the clinical stage, which may materially and adversely impact our business.
All of our R&D programs are in the early, pre-application stage of preclinical development and our future success heavily depends on the successful development of our DehydraTECH product candidates, which may never occur. These product candidates could be delayed, not advance into the clinic, or unexpectedly fail at any stage of development. Before we can commence clinical trials for a product candidate, we must conduct extensive preclinical and other non-clinical tests in order to support an investigational new drug (“IND”) application, including IND-enabling good laboratory practice toxicology studies, in the United States or their equivalents with regulatory authorities in other jurisdictions. Preclinical studies and clinical trials are expensive, difficult to design and can take many years. There is no assurance that we will be able to successfully develop our product candidates, and we may focus our efforts and resources on product candidates that may prove to be unsuccessful.
We cannot be certain of the outcome of preclinical testing and clinical studies and results from these studies may not predict the results that will be obtained in later phase trials of our product candidates. Even if we are able to complete our preclinical studies and planned clinical trials in line with our projected timelines, results from such studies and trials may be not replicated in subsequent preclinical studies or clinical trial results. Additionally, such studies may be delayed due to events beyond our control including as a result of natural disasters of any kind. As a result, we cannot guarantee that we will be able to submit INDs, or similar applications, within our projected timelines, if at all, or that the FDA, or similar regulatory authorities, will allow us to commence clinical trials.
Pharmaceutical products incorporating DehydraTECH has never been approved for the treatment of disease.
In order to commercialize a product that utilizes DehydraTECH for the treatment of any disease, we and/or our commercial partner must obtain regulatory approvals for such product for treatment of a particular indication. Satisfying regulatory requirements is an expensive process that typically takes many years and involves compliance with requirements covering R&D, testing, manufacturing, quality control, labeling, and promotion of drugs for human use. To obtain necessary regulatory approvals, a licensee must, among other requirements, complete clinical trials demonstrating that their product is safe and effective for a particular indication. There can be no assurance that any product enhanced by DehydraTECH will be proven to be safe and effective, that the clinical trials will demonstrate the necessary safety and effectiveness of the product candidates, or that we will be successful in obtaining regulatory approval for any treatment developed, even if such safety and effectiveness are demonstrated.

| Page 32 of 90 |
| Table of Contents |
Any delays or difficulties encountered in such clinical trials may delay or preclude regulatory approval from the United States Food and Drug Administration (the “FDA”) or from international regulatory organizations. Any delay or preclusion of regulatory approval would be expected to delay or preclude the commercialization of their product that utilizes DehydraTECH. Examples of delays or difficulties that may be encountered during clinical trials include without limitation the following:
| · | clinical trials may not yield sufficiently conclusive results for regulatory agencies to approve the use of DehydraTECH; |
| · | DehydraTECH enhanced formulations may fail to be more effective than current therapies, or to be effective at all; |
| · | DehydraTECH enhanced formulations may have adverse side effects, which could cause them to be delayed or precluded from receiving regulatory approval or otherwise expose us to significant commercial and legal risks; |
| · | it may take longer than expected to determine whether or not a treatment is effective; |
| · | patients involved in the clinical trials may suffer severe adverse side effects even up to death, whether as a result of treatment with DehydraTECH enhanced formulations, the withholding of such treatment, or other reasons whether within or outside of our control; |
| · | failure to be able to enroll a sufficient number of patients in the clinical trials; |
| · | patients enrolled in the clinical trials may not have the characteristics necessary to obtain regulatory approval for a particular indication or patient population; |
| · | failure to obtain and/or maintain, any required governmental approvals; |
| · | if approval for commercialization is granted, it is possible the authorized use will be more limited than is necessary for commercial success, or that approval may be conditioned on completion of further clinical trials or other activities, which will cause a substantial increase in costs; |
| · | if granted, approval may be withdrawn or limited if problems with DehydraTECH enhanced formulations emerge or are suggested by the data arising from their use or if there is a change in law or regulation. |
Any success achieved at a given stage of the clinical trials does not guarantee that the future achievement of success at any subsequent stage, including without limitation, final FDA approval.
Delays or rejections in the regulatory approval process because of additional government regulation resulting from future legislation or administrative action, or from changes in the policies of the FDA or other regulatory bodies during the period of product development, clinical trials, or regulatory review may occur. Failure to comply with applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production, or an injunction preventing certain activity, as well as other regulatory action against our product candidates or us.
We currently have no commercial pharmaceutical products and therefore generate no revenue from pharmaceutical products and may never be able to develop marketable pharmaceutical products. We have no experience in filing the applications necessary to obtain marketing approval and expect that we will need to rely on CROs and regulatory consultants to assist us with this process. Regulatory approval also requires the submission about the product manufacturing process and inspection of the manufacturing facilities, to the relevant regulatory authority.
Our success is dependent on our, or our licensee’s, ability to successfully navigate the risks and obstacles associated with obtaining FDA clearance for any DehydraTECH enhanced formulated product

| Page 33 of 90 |
| Table of Contents |
(ii) Risks related to regulatory and development approvals for pharmaceutical products
Pharmaceutical products using DehydraTECH with CBD as an API have never been approved for the treatment of any disease.
Some of the pharmaceutical product candidates that we intend to develop may contain CBD and/or THC. To date the FDA has approved only limited use of cannabinoids for the treatment of any disease or condition. The FDA has approved one cannabinoid-derived drug product for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome and three synthetic cannabinoid-related drug products for the treatment of nausea and vomiting caused by cancer chemotherapy. While we expect any product candidates that we develop will be regulated as a new drug under the Federal Food, Drug, and Cosmetic Act, the FDA could decide to regulate them or any other products incorporating DehydraTECH under a different regulatory regime. The lack of policies, practices or guidelines may hinder or slow review by the FDA of any regulatory filings that we may submit. Moreover, the FDA may respond to these submissions by defining requirements that we may not have anticipated.
(iii) Risks related to controlled substances
Hemp-based CBD can be confused with marijuana-based CBD which remains illegal under federal law.
In conjunction with the enactment of the Agriculture Improvement Act of 2018 (the “Farm Bill”), the FDA released a statement about the status of CBD as a nutritional supplement, and the agency’s actions in the short term with regards to CBD will guide the industry. The regulation of CBD products is currently in constant flux and any difficulties in compliance with future government regulation could increase our operating costs and adversely impact our results of operations in future periods. Furthermore, violations of these laws, or alleged violations, could disrupt our business or the business of our licensees and result in a material adverse effect on our operations. We cannot predict the nature of any future laws, regulations, interpretations, or applications, and it is possible that regulations may be enacted in the future that will be directly applicable to our business.
In addition, the interstate shipment of hemp-derived CBD from one state to another is legal only where both states have laws and regulations that allow for the production and sale of such products and that qualify under the Farm Bill. Therefore, the marketing and sale of DehydraTECH products containing hemp-derived CBD is limited by such factors and is restricted to such states. A repeal or adverse amendment of laws and regulations that are now favorable to the distribution, marketing, and sale of finished products our licensees intend to sell could significantly limit, restrict or prevent us from generating revenue related to DehydraTECH technology-enabled products that contain hemp-derived CBD. Any such repeal or adverse amendment of now favorable laws and regulations could have an adverse impact on our business plan with respect to such revenues
Controlled substance legislation differs between counties, states and countries and legislation in certain countries may restrict or limit our ability to develop and commercialize products using DehydraTECH.
We currently have licensees who produce hemp-derived CBD products. The Farm Bill delegates the authority to the states to regulate and limit the production of hemp and hemp-derived products within their territories. Although many states have adopted laws and regulations that allow for the production and sale of hemp and hemp-derived products under certain circumstances, no assurance can be given that such state laws may not be repealed or amended such that our intended products containing hemp-derived CBD would once again be deemed illegal under the laws of one or more states now permitting such products, which in turn would render such intended products illegal in those states under federal law even if the federal law is unchanged. In the event of either repeal of federal or of state laws and regulations, or of amendments thereto that are averse to our or our licensee’s products, we may be adversely impacted with respect to DehydraTECH-enabled CBD product revenue or royalties.

| Page 34 of 90 |
| Table of Contents |
Although Lexaria does not sell any marijuana or marijuana-based CBD, under its discontinued business operations, its former licensee’s products could be treated as being illegal under federal or state authorities.
Lexaria has discontinued business operations which had ancillary involvement exposure via out-licensing of its intellectual property to licensees that may utilize DehydraTECH in the production of products that contain contents which are locally or state approved but federally controlled. Where licensee’s products contain controlled contents any revenue streams from such licensee’s may be interrupted by regulatory involvement in their business.
D. Risks Associated with Securities Markets and Ownership of our Common Stock
(i) Risks related to pricing volatility of common stock and warrants
The trading price of the shares of our common stock could be highly volatile and as such investors could incur substantial losses.
Prospects for companies in the biotechnology industry may be regarded generally as uncertain given the nature of the industry and, accordingly, investments in biotechnology companies should be regarded as speculative. We have experienced erratic share-price and trading volume movement of our common stock which could be influenced by any number of factors which include the Risk Factors discussed in this section of the Report on 10-K and many others. In general, trading stocks on any market and particularly in stocks of bioscience companies can be characterized by wide fluctuations in trading prices, due to many factors that may be unrelated to the operating performance or business prospects of any particular company.
We have warrants that are listed on the Nasdaq pursuant to our January 2021 underwritten offering but they do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price. Upon exercise of a warrant, a holder will be entitled to exercise the rights of a common stockholder as to the security exercised only as to matters for which the record date occurs after the exercise. Although the warrants from the Company’s underwritten offering are currently trading on Nasdaq, there can be no assurance that there will be an active trading market for the warrants. Without an active trading market, the liquidity of the warrants will be limited.
(ii) Risks related to strategic transactions
Our by-laws do not contain anti-takeover provisions, which could result in a change of our management, directors and directors if there is a take-over of our company.
We do not currently have a shareholder rights plan or any anti-takeover provisions in our by-laws. Without any anti-takeover provisions, there is no deterrent for a take-over of our Company, which may result in a change in our management and/or directors.

| Page 35 of 90 |
| Table of Contents |
(iii) Risks related to non-payment of dividends and dilution
Because we do not intend to pay any dividends on our shares, investors seeking dividend income or liquidity should not purchase our shares.
We have not declared or paid any dividends on our shares since inception, and do not anticipate paying any such dividends for the foreseeable future. We presently do not anticipate that we will pay dividends on any of our common stock in the foreseeable future. If payment of dividends does occur at some point in the future, it would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any common stock dividends will be within the discretion of our Board of directors. We presently intend to retain all earnings to implement our business plan; accordingly, we do not anticipate the declaration of any dividends for common stock in the foreseeable future. Investors seeking dividend income or liquidity should not invest in our shares.
Because we can issue additional shares, purchasers of our shares may incur immediate dilution and may experience further dilution.
We are authorized to issue up to 220,000,000 shares. The board of directors of our Company has the authority to approve additional share issuances, and to determine the rights, preferences, and privileges of such shares, without consent of any of our stockholders. Consequently, our stockholders may experience more dilution in their ownership of our Company in the future.
(iv) Risks related to smaller reporting company compliance
We are a “smaller reporting company” under the SEC’s disclosure rules and have elected to comply with the reduced disclosure requirements applicable to smaller reporting companies.
We are a “smaller reporting company” under the SEC’s disclosure rules, meaning that we have either:
| · | a public float of less than $250 million; or |
| · | annual revenues of less than $100 million during the most recently completed fiscal year; and |
| ○ | no public float; or |
| ○ | a public float of less than $700 million. |
As a smaller reporting company, we are permitted to comply with scaled-back disclosure obligations in our SEC filings compared to other issuers, including with respect to disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We have elected to adopt the accommodations available to smaller reporting companies. Until we cease to be a smaller reporting company, the scaled-back disclosure in our SEC filings will result in less information about our company being available than for other public companies. If investors consider our common shares less attractive as a result of our election to use the scaled-back disclosure permitted for smaller reporting companies, there may be a less active trading market for our common shares and our share price may be more volatile.
We are also a non-accelerated filer under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and we are not required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. Therefore, our internal controls over financial reporting will not receive the level of review provided by the process relating to the auditor attestation included in annual reports of issuers that are subject to the auditor attestation requirements. In addition, we cannot predict if investors will find our common shares less attractive because we are not required to comply with the auditor attestation requirements. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and trading price for our common shares may be negatively affected.

| Page 36 of 90 |
| Table of Contents |
Operating as a public company, we incur increased costs and our management is required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company we have incurred, and will continue to incur, significant legal, accounting, and other fees related to our compliance measures under the listing requirements of SEC, Nasdaq, the Sarbanes-Oxley Act of 2002 ("Sarbanes-Oxley"), the Dodd-Frank Wall Street Reform, British Columbia Securities Commission, Ontario Securities Commission, FINRA and other applicable securities rules and regulations. Our management devotes a substantial amount of time towards maintaining compliance with these requirements including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. These requirements increase our legal and financial compliance costs and make some activities more time-consuming and costly. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. The increased costs could impact our results of operations, and may require us to reduce costs in other areas of our business or increase the prices of our products or services. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements and other requirements could also make it more difficult for us to attract and retain qualified persons to serve on our Board of directors, our board committees, or as executive officers.
E. General Risks
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with their procedural, documentary, fee payment and other provisions during the patent application process. Periodic maintenance fees on issued patents often must be paid to the USPTO and foreign patent agencies over the lifetime of each patent. While an unintentional lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications covering our intellectual property, we may not be able to stop a competitor from utilizing our Technology, which would have a material adverse effect on our business.

| Page 37 of 90 |
| Table of Contents |
We face risks related to our collection and use of data, disruptions or failures of our information technology systems or breaches of information security that could adversely affect our business and operations.
Our internal computer systems and those of our CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized CRO access, telecommunication and electrical failures, and natural disasters. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our R&D programs. We depend on digital technologies for the successful operation of our business, including corporate email communications to and from employees, licensees, consultants and third-party providers, collection, use and retention of investor data, security systems with respect to our Health Canada licensed laboratory and maintenance of confidential information.
As part of our business model, we collect, retain, and transmit confidential information over public networks. We have enterprise class and industry comparable security measures in place to protect both our physical facilities and digital systems from attacks. Despite these efforts, however, we may be vulnerable to targeted or random personal data or security breaches, acts of vandalism, computer malware, misplaced or lost data, programming and/or human errors, or other similar events. Awareness and sensitivity to personal data breaches and cyber security threats is at an all-time high. Any misappropriation of confidential or personal information gathered, stored or used by us, be it intentional or accidental, could have a material impact on the operation of our business, including severely damaging our reputation and our relationships with our licensees, employees and investors. We may incur further significant costs implementing additional security measures to protect against new or enhanced data security or privacy threats, or to comply with current and new international, federal, and state laws governing the unauthorized disclosure of confidential and personal information which are continuously being enacted and proposed. We could also experience loss of revenues resulting from unauthorized use of proprietary information including our intellectual property. We could also face sizable fines, significant breach containment and notification costs to supervisory authorities and the affected data subjects, and increased litigation as a result of cyber security or personal data breaches.
If we are unable to hire and retain qualified personnel, we may not be able to implement our business plan successfully.
In developing DehydraTECH, we rely upon our employees, consultants, contractors, and collaborators. Our current business prospects are dependent on the principal members of our executive team, the loss of whose services could make it difficult for us to manage our business successfully and achieve our business objectives. Our ability to identify, attract, integrate, and retain additional qualified key personnel is critical to our success. Competition for skilled research, product development, regulatory and technical personnel is intense, and we may not be able to recruit and retain the personnel we need. The loss of the services of any key research, product development, regulatory and technical personnel, or our inability to hire new personnel with the requisite skills, could restrict our ability to carry out our R&D programs and/or develop our product candidates. Because we are a smaller reporting entity, the loss of any key personnel could result in more severe disruption to our operations than it would to a larger company, since of necessity each person in a small company carries relatively greater duties responsibilities than that person would in a larger company.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed alleged trade secrets.
We employ, and may employ in the future, individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors which is common in the biotechnology and pharmaceutical industries. Although we have policies that dissuade our employees, consultants and independent contractors in the use of any proprietary information or know-how of their previous employers in their employment with us, we could be subject to claims that the Company or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims and the failure to defend against such claims, could result in the loss of valuable intellectual property rights or personnel in addition to suffering monetary damages. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management and which could adversely impact our business.

| Page 38 of 90 |
| Table of Contents |
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Description of Property
Our executive offices and research lab are located in a leased facility in Kelowna, British Columbia Canada, consisting of approximately 2,250 square feet of office space to accommodate our finance and administrative functions as well as approximately 1,000 square feet of laboratory space accommodating our in-house research and development. The lease for our Kelowna offices commenced in November 2019 and is scheduled to expire November 2023. Subject to the terms and conditions of the lease, we may extend the term of the lease for another five years. Our facilities are in excellent condition and adequate for their current use.
Item 3. Legal Proceedings
We know of no other material, existing or pending legal proceedings against our Company, nor are we involved as a plaintiff in any other material proceeding or pending litigation. There are no other proceedings in which any of our directors, executive officers, or affiliates, or any registered or beneficial stockholder, is an adverse party or has a material interest adverse to our interest.
Item 4. Mine Safety Disclosures
Not Applicable.

| Page 39 of 90 |
| Table of Contents |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Effective January 12, 2021, the Company’s common stock and warrants began trading on the Nasdaq Capital Markets under the symbols “LEXX” and “LEXXW”, respectively. Prior to this date the Company’s common stock was quoted on the OTCQX under the symbol “LXRP.” Our common shares were also quoted on the Canadian Securities Exchange under the symbol “LXX” until July 8, 2021. Since Lexaria's shares began trading on the Nasdaq, the overwhelming majority of trading has moved to Nasdaq, providing greater liquidity for shareholders. The Company expects to realize savings in fees and managerial time and effort that were required to maintain a dual listing that can now be redirected into the Company's applied research and development programs, further advancing the business of the Company. For these reasons, Lexaria's management team and Board of directors have made the decision to voluntarily delist from the CSE, consolidating the trading of its shares to Nasdaq.
The stock market in general has experienced extreme stock price fluctuations in the past few years. In some cases, these fluctuations have been unrelated to the operating performance of the affected companies. Many companies have experienced dramatic volatility in the market prices of their common stock. The Company believes that several factors, both within and outside of its control, could cause the price of the Company’s common stock to fluctuate.
The following quotations, obtained from Yahoo Finance, reflect the high and low bids, up to the first quarter of our 2021 fiscal year, for our common shares as quoted on the OTCQX and Nasdaq stock markets, based on inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions. Thereafter, the high and low bids for the balance of the 2021 fiscal year represent trades of our common shares on the Nasdaq Capital Markets.
All share and per-share amounts presented have been retroactively adjusted for all periods represented to reflect the 1-for-30 reverse stock split effected January 11, 2021.
FY 2021 |
| Bid Price |
| |||||
|
| High |
|
| Low |
| ||
First quarter |
| $ | 10.79 |
|
| $ | 5.10 |
|
Second quarter |
| $ | 10.50 |
|
| $ | 3.98 |
|
Third quarter |
| $ | 8.85 |
|
| $ | 4.50 |
|
Fourth quarter |
| $ | 12.50 |
|
| $ | 5.29 |
|
FY 2020 |
|
|
|
|
|
|
|
|
First quarter |
| $ | 22.80 |
|
| $ | 12.00 |
|
Second quarter |
| $ | 17.10 |
|
| $ | 9.11 |
|
Third quarter |
| $ | 13.42 |
|
| $ | 6.54 |
|
Fourth quarter |
| $ | 15.64 |
|
| $ | 7.11 |
|
There were 5,726,699 common shares issued and outstanding as of August 31, 2021 (3,001,476 at August 31, 2020). As of November 25, 2021 there were approximately [49] shareholders of record.
Dividend Policy
We have not paid any and have no present intention of paying any dividends on our capital stock. Our current policy is to retain earnings, if any, for use in our operations and in the development of our business. As a result, we anticipate that only appreciation of the price of our common stock, if any, will provide a return to investors for at least the foreseeable future.
Recent Sales of Unregistered Securities
Other than set out below, we did not sell any equity securities which were not registered under the Securities Act during the year ended August 31, 2021, that were not otherwise disclosed on our quarterly reports on Form 10-Q or our current reports on Form 8-K filed during the year ended August 31, 2021. A summary of the activity is set out in the table below:
Type of Issuance |
| Number of Shares |
|
| Total Value $ |
| ||
Per agreements(1) |
|
| 12,178 |
|
|
| 85,000 |
|
|
|
| 12,178 |
|
|
| 85,000 |
|
(1) The Company awarded the restricted common shares as required by consulting contracts.

| Page 40 of 90 |
| Table of Contents |
Warrants
There were 610,189 warrants at a strike price of $6.58 exercised during the year ended August 31, 2021.
Equity Compensation Plan Information
We have no long-term incentive plans other than the equity incentive plan described below.
Equity Incentive Plan
During the year ended August 31, 2021, the Company cancelled its 2014 Stock Option Plan. The 2007 and 2010 option plans were cancelled during the year ended August 2020. All future option issuances shall be made under the Equity Incentive Plan.
The Board may, subject to the approval of any regulatory authority whose approval is required, amend, suspend or terminate this Plan or any portion thereof. No such amendment, suspension or termination shall alter or impair any outstanding unexercised Options or any rights without the consent of such Participant. If this Plan is suspended or terminated, the provisions of this Plan and any administrative guidelines, rules and regulations relating to this Plan shall continue in effect for the duration of such time as any Option remains outstanding.
Securities authorized for issuance under equity compensation plans | |||
Plan Category | Number of securities to be based upon exercise of outstanding options, warrants and rights | Weighed-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plan [excluding securities reflected in column (a)] |
| (a) | (b) | (c) |
Equity compensation plans not approved by shareholders | Nil | Nil | Nil |
Equity compensation plans approved by shareholders | 206,170 | 7.36 | 304,263 |
Total | 206,170 | 7.36 | 304,263 |
Convertible Securities
During the year ended August 31, 2021, pursuant to the Equity Incentive Plan the Company granted stock options to directors, officers, employees, and consultants that enable the option holders to purchase an aggregate of up to 84,900 common shares of the Company at prices of: 3,400 at $4.80, 38,500 at $5.31, 26,000 at $5.83, 5,000 at $5.31 vesting after one year and 12,000 at $5.04 vesting over three years. All options have a 5-year term. The 159,835 options vested have a fair value of $328,801 using the Black Scholes valuation method and were included in consulting and wages expense during the year.

| Page 41 of 90 |
| Table of Contents |
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
We did not purchase any of our shares of common stock or other securities during our fiscal year ended August 31, 2021.
Item 6. Selected Financial Data
As a “Smaller Reporting Company”, this Item and the related disclosure is not required.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
This discussion and analysis contains forward-looking statements that involve not only risks and uncertainties but also changes in condition, significance, value and other factors, as described in “Risk Factors” and elsewhere in this Annual Report on Form 10-K, that could cause our actual results of operations, performance, financial position and business prospects and opportunities for this fiscal year and the periods that follow to differ materially from those expressed in, or implied by, those forward-looking statements. This discussion and analysis should be read in conjunction with our consolidated financial statements and the accompanying notes related thereto that appear elsewhere in this Annual Report on Form 10-K.
The following management’s discussion and analysis of financial condition and results of operations (“MD&A”) is provided to enhance the readers understanding of our results of operations and financial condition for the year ended August 31, 2021, and in comparison, to the year ended August 31, 2020.
Executive Summary
Lexaria’s patented technology DehydraTECH improves the delivery of bioactive compounds while promoting healthy ingestion methods, lowers overall dosing, and is highly effective in active molecule delivery available in a range of formats from oral ingestible to oral buccal/sublingual to topical products. DehydraTECH substantially improves the rapidity and quantity of API transport to the blood plasma and brain using the body’s natural process for distributing fatty acids via the oral route. This technology extends across many categories beyond the primary pharmaceutical focus of the Company from foods and beverages to cosmetic products and nutraceuticals.
Lexaria is advancing a several R&D activities in both preclinical and clinical programs. Currently, our program is investigating cannabidiol (CBD) for the reduction of hypertension with three human clinical trials during calendar 2021, and one human clinical trial planned during calendar 2022. Other programs include nicotine for oral pouches and nicotine replacement therapy, antivirals and related compounds for COVID-19 and other viral diseases, PDE5 inhibitors, NSAIDS, hormones, and others. From time to time the Company will engage in contract R&D for third parties who are interested in in evaluating DehydraTECH in their products.
Evaluate the financial condition and operating performance
Fiscal 2021 was highlighted by the intensified direction of our research and development programs as we scale up our research based on the continued confirmatory results from our ongoing programs. During the year ended August 31, 2021, we completed ten studies and initiated a further seven. These programs have been supported by the capital infusion as Lexaria raised approximately $15m in funding during the year which has enabled the active work programs of 2021 and supports significant advance in the fields of heart disease and hypertension, oral nicotine, and antiviral research.

| Page 42 of 90 |
| Table of Contents |
We consider advancing our applied R&D studies as a vital step towards our goal of establishing commercial relationships with potential industry partners who can utilizes DehydraTECH within our existing or new product lines. We continue to conduct additional in vitro and in-vivo studies testing the absorption of some or all of the molecules named within our patent applications – CBD, NSAIDs, vitamins, PDE5 inhibitors, nicotine and anti-viral drugs – to further substantiate the effectiveness of DehydraTECH. Successful tests are expected to increase awareness and acceptance of DehydraTECH as a meaningful method by which to deliver some or all of the named molecules more effectively than current delivery methods avail. Therefore, absorption tests are an important element leading towards higher rates of acceptance and implementation of our technology licensing initiatives.
We will pursue technology licensing opportunities as a method of generating highly profitable revenue streams over long periods of time. In addition, while nine of our US patents and eight of our Australian patents have been granted to date, we have multiple other applications filed in the US and around the world. It is not possible to forecast with certainty when, or if, our remaining patents pending will become granted patents. But if our remaining patent applications do become granted patents, our ability to generate meaningful license revenue from our intellectual property may increase in a short period of time.
Lexaria is debt free and expects its current cash reserves to meet all its needs for the twelve months following the release of this report. As such the budget for applied R&D during fiscal 2022 is fully funded. The Company plans to seek strategic corporate business partners for many of its specific drug investigations after sufficient data has been generated which, if successful, could generate any combination of up-front milestone and/or royalty payments to the Company.
We will continue to pursue patent protection in more than 40 countries around the world as vigorously as we are able, since the successful granting of more of those applications could lead to material increases in shareholder value.
We expect to devote an increasing proportion of our resources and focus towards pharmaceutical applications and launched operations in this division during the 2022 fiscal year. Our past R&D in other sectors has contributed greatly to our understanding of DehydraTECH and has encouraged us to attempt to reach more lucrative commercial applications in the pharmaceutical sector
We continue to communicate the benefits of DehydraTECH to potential licensing partners, i.e. with higher absorption levels a manufacturer could perhaps infuse smaller amounts of active molecules into a product, thus reducing their manufacturing input costs, to provide higher bioavailability with the dosing limits being imposed or contemplated in many jurisdictions, to infuse consumer products while masking the flavor and smell of the active molecules, and predictable delivery times. We believe these to be meaningful competitive advantages that may lead to the potential to generate licensing revenue, and will pursue these opportunities within the cannabinoids, nicotine and other bioactive molecular markets both within the USA and also internationally, in those locations where they are legal and regulated by government.
Asset Sale
On December 9, 2020, Lexaria CanPharm ULC (“CanPharm”) completed a disposition (the “Disposition”) of its use and licensing rights to use its DehydraTECH technology (the “Assets”) specifically in association with non-pharmaceutical products containing cannabis molecules that contain 0.3% or greater THC. The purpose of the Disposition was to remove the Company’s association with cannabis as it remains a Schedule 1 Drug and thereby eliminating any such regulatory restrictions cannabis products may create. The Disposition assisted the Company in obtaining a listing on the Nasdaq Capital Market (“Nasdaq”) on January 12, 2021. As a result of the Disposition, CanPharm assigned to the purchaser Hill Street license agreements with three existing non-related party licensees.

| Page 43 of 90 |
| Table of Contents |
In consideration for the Assets, Hill Street provided CanPharm with C$350,000 cash, a promissory note bearing a principal amount of C$2,000,000 and bearing an interest rate of 10% (the “Note”) and C$1,500,000 in shares of Hill Street, issuable in three tranches by April 9, 2022. The repayment of the Note does not have a fixed maturity date and is based on quarterly installments equal to 5% of the gross sales realized by Hill Street of DehydraTECH enabled products. Due to the uncertainty pertaining to the settlement of the Note, management concluded that the note had $NIL value at the time of the sale and was recorded as such. Some of the factors considered in the $Nil valuation of the Note were that the legal sales of THC products in the US and Canada have little or no history which made the expectant quarterly payments very difficult to forecast. Further, Hill Street had no experience selling THC products and at the time of the sale was not licenced to produce and sell such products. Therefore, the Company considered risk of default high and the collectability of the Note as highly doubtful. Since the date of sale Hill Street has repaid $4,854 in the year-ended August 31, 2021. Subsequent to fiscal 2021, the Company has received a further $6,632 payment toward the balance of the Note. These amounts are considered interest income when received.
Reverse Stock Split
On January 11, 2021, the Company filed an amendment and restatement of its articles of incorporation to effectuate a 1-for-30 reverse stock split of the issued and outstanding shares of common stock of the Company. The purpose of the reverse stock split was to meet Nasdaq’s minimum stock price requirement. The reverse stock split did not change the number of authorized shares of common stock, which remains at 220,000,000 shares. All warrants, options, share and per share information in this Report gives retroactive effect to the 1-for-30 reverse stock split.
Public Offering
On January 14, 2021, the Company closed an underwritten public offering with the issuance of 2,102,856 shares of the Company’s common stock price of $5.25 per share with an equivalent number of five-year warrant at an exercise price of $6.58. Additionally, 227,161 Representative Warrants were issued as partial consideration to the underwriters of the offering that have a five-year term at an exercise price of $6.58. Net of fees and disbursements, the Company received net proceeds of $9,471,497. The Company plans to use approximately $3,700,000 of the net proceeds for research and development studies and the patent and legal costs associated thereto, with the remaining net proceeds to be used for general working capital purposes.
LEXX Market Listing
The Company’s common stock was uplisted from trading on the OTCQX under “LXRP” to the Nasdaq Capital Market where our common stock and warrants began trading under the symbols “LEXX” and “LEXXW”, respectively, effective as of the opening of market trading on January 12, 2021.
The Company, trading under the symbol “LXX”, voluntary delisted from the Canadian Securities Exchanges (“CSE”) effective after the closing of trading on Wednesday, July 7, 2021. The overwhelming majority of trading has moved to Nasdaq and by delisting from the CSE the Company expects to realize savings in fees and managerial time and effort that had been required to maintain a dual listing.

| Page 44 of 90 |
| Table of Contents |
Results of Operations for our Year Ended August 31, 2021
Our net loss from operations for the year ended August 31, 2021, was $5,686,852 (2020 - $4,084,613). The changes between these periods for the respective items are summarized as follows:
|
| August 31 |
|
| August 31 |
|
|
|
| |||
|
| 2021 |
|
| 2020 |
|
| Change |
| |||
|
| $ |
|
| $ |
|
| $ |
| |||
Revenue |
|
| 722,738 |
|
|
| 384,543 |
|
|
| 338,195 |
|
Research & development |
|
| 1,262,895 |
|
|
| 387,074 |
|
|
| 875,821 |
|
Consulting fees & employees |
|
| 2,627,765 |
|
|
| 2,594,359 |
|
|
| 33,406 |
|
Legal and professional |
|
| 703,407 |
|
|
| 450,494 |
|
|
| 252,913 |
|
General and administrative |
|
| 1,640,177 |
|
|
| 917,958 |
|
|
| 722,182 |
|
Net operating loss |
|
| (5,686,852 | ) |
|
| (4,084,613 | ) |
|
| (1,602,222 | ) |
Revenue
Lexaria’s business operations include technology licensing agreements wherein corporate licensees implement DehydraTECH under license within our facilities under royalty agreements and also includes corporate clients that purchase pre-processed DehydraTECH CBD-powders manufactures at a Lexaria -contracted GMP-certified food facility for shipment back to the client for integration into their final product formats. Fees payable to the Company contain a mixture of both manufacturing charges as well as royalty and trademark fees.
The primary source of revenues for the Company are derived from Lexaria Hemp where sales of B2B processing of intermediary product saw an increase of approximately 153% (2021 - $383,179, 2020 – $151,634) in the year and contributed approximately 53% of the 2021 annual revenues.
Lexaria developed a line of demonstration oral-delivered products that were utilized to show the efficacy of DehydraTECH and enabled the ability of manufacturers to incorporate the technology into their product lines. We earlier offered these products for sale to consumers through our web-based sales platform. During the year-ended August 31, 2021 we discontinued these direct-to-consumer demonstration products and closed our web sales platform in order to intensify our efforts on B2B production.
During the year the Company sold the underlying assets of its THC-related business to Hill Street, a Canadian company that is now producing and selling THC infused products using the DehydraTECH technology in Canada with planned expansion into the US. Lexaria’s gross revenues from this discontinued operation were $3,000 in fiscal 2021, and $69,750 in fiscal 2020.
Licensing revenues, particularly usage fees, increased more than 40% in the year ended August 31, 2021 (2021 $334,974 – 2020 $232,909) and correspond in part to the increased B2B product sales. Licensing revenues generally deliver much higher gross profit margins than do product revenues. During the year ended August 31, 2021, the Company also generated $86,921 (2020- $Nil) from R&D contracts.
During the year ended August 31, 2021, the Company renegotiated a contract with one of our existing licensees who held an exclusive territorial use of our DehydraTECH technology. Due in part to logistical constraints, the customer has agreed to relinquish territorial exclusivity and has continued to use our technology under licence. Revenues of $101,000 were conceded by the Company in the revision of terms.
In fiscal 2022 the Company expects to derive increased revenues from technology licensing to third parties as market demand for Hemp based products increase and supply chain logistics improve. The expansion of our intellectual property portfolio and conducting supportive R&D will jointly contribute to strengthening revenue prospects.

| Page 45 of 90 |
| Table of Contents |
Research and Development
Research and development costs are expensed as incurred and account for a significant portion of our operational expenses. With proceeds from our underwritten public offering in January of 2021, we were able to direct additional expenditures to the increased focus on studies pertaining to hypertension and anti-viral drugs. We plan to continue to invest in our R&D programs for the foreseeable future and we expect these expenses will increase in 2022 compared to 2021. Our R&D programs will continue to be directed at four core business segments; heart disease including hypertension, reduced-risk non-combusted nicotine, improve antiviral drug delivery and CBD from hemp. Of significant note, we are in the late stage planning of an initiation of Investigational New Drug (“IND”) trials for DehydraTECH in the US during fiscal 2022. Preclinical and clinical development is inherently unpredictable as is regulatory approval and commercialization, therefore we are unable to estimate with any certainty the costs we will incur and the timelines required in our continued development and commercialization efforts. Any successful development and completion of clinical trials as well a regulatory approval and commercialization are uncertain and may not result in approved products. Completion dates and completion costs can vary significantly for each future product candidate and are difficult to predict. Lexaria and our commercial partners will continue to explore multiple R&D programs directed toward further evaluation, development, and commercialization of our DehydraTECH technology.
General and Administrative
General and administrative expense consists primarily of consulting fees and personnel in executive, accounting, and other administrative functions as well as advertising and marketing, investor relations and stock-based compensation expense. General and administrative expense also includes corporate facility costs, including rent and utilities, insurance premiums, legal fees related to corporate matters, and fees for auditing, accounting, and other consulting services.
Our general and administrative expenses saw an overall increase of $988,645 during the year ended August 31, 2021, from $3,982,704 for the prior year ended August 31, 2020. In effort to bring the results of the Company’s R&D programs to the attention of various industry sectors and to the scientific and investment communities, the Company accelerated its advertising, promotion, and investor relations programs. Lexaria participated in 5 virtual investor conferences during the year and issues press releases on a regular basis designed to provide continuous disclosure. This marketing outreach program resulted in increased spending of $441,114 for a total in the year ended August 31, 2021, of $829,668 ($388,554 – August 31, 2020). Licensing, filing, and regulatory fees increased by $135,229 due to additional fees for SEC filings corresponding to our listing on the Nasdaq exchange in January 2021.
Included in general and administration expenses in the year ended August 31, 2021, is a cumulative unrealized net-loss on marketable securities of $166,255. The unrealized loss is attributable to shares received as a part of the sale of assets in the year. Management has concluded that the loss is likely temporary in nature based on our evaluation of available information.
Our consulting fees, included in general and administrative expenses, decreased by $24,575 in the year ended August 31, 2021, primarily due to the higher non-cash payments for services included in fiscal 2020 derived from the granting of options. Our executive compensation is typically categorized under consultant fees and costs excluding non-cash share-based payments associated with those agreements comprise a significant portion of our expenditures on consulting fees.

| Page 46 of 90 |
| Table of Contents |
Included in general and administration expenses, legal and professional fees saw an increase of $148,270 to $703,407 during the year primarily related to securities, patent, and trademark related filings and other advisory services. Accounting and auditing fees also increased by approximately 75% ($58,295) year over year. We recognize certain accounting and professional tax advisory services as “Professional Fees”. During fiscal 2021 Lexaria was granted three additional patents in the US, India and in Japan. We have over 50 patents pending internationally. Although we endeavour to minimize expenses, when possible, we consider that increased costs related to patent and trademark work reflects positive progress in attempting to build the value of our intellectual property portfolio, and in executing our business plan.
Corporate general and administrative expenses are expected to increase moderately in fiscal 2022 as compared to 2021 as a result of higher human resource, regulatory, legal and investor relations costs and the potential impact of inflation.
Liquidity and Capital Resources
Since Lexaria’s entry into the bioscience sector in 2015 and through to August 31, 2021, we have accumulated a $23.5m deficit despite generating total gross revenues of $1.9m. We have used the issuance of common shares to raise the required capital to fund our expenditures. Since fiscal 2014, we have raised an aggregate of $25.3 m to fund our operations, of which $16.1 m was from the sale of our common stock, $8.8 m from warrants and $0.4 m were proceeds from the exercise of stock options. We may offer additional securities for sale during our fiscal year 2022 or thereafter in response to market conditions or other circumstances if we believe such a plan of financing is required to advance the Company’s business plans and is in the best interests of our stockholders. There is no certainty that equity or debt financing will be available in the future or that it will be at acceptable terms and at this time, it is not possible to predict the outcome of these matters.
We have incurred significant net losses of approximately $4.2 m and $4.1 m for the two years ended August 31, 2021, and August 31, 2020, respectively. We expect to continue to incur significant operational expenses and net losses in the upcoming 12 months and beyond. Our net losses may fluctuate significantly from quarter to quarter and year to year, depending on the stage and complexity of our R&D studies and related expenditures, the receipt of additional payments on the licencing of our technology, if any, and the receipt of payments under any current or future collaborations we may enter into.
The Company has evaluated whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company's ability to continue as a going concern. As of August 31, 2021, the Company had cash and cash equivalents of approximately $10.9 m to settle $153,276 of current liabilities and thus the Company believes this will enable the Company to fund its operating and R&D expenses requirements through at least one year from the issuance date of this report. The Company does not anticipate making any material capital expenditures in the fiscal 2022 as we believe our facilities and equipment held at the year ended August 31, 2021, are sufficient for at least twelve months proceeding the date of filing this report.
|
| August 31 |
|
| August 31 |
| ||
Working Capital |
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
Current assets |
|
| 12,442,940 |
|
|
| 1,925,961 |
|
Current liabilities |
|
| (153,276 | ) |
|
| (225,917 | ) |
Net Working Capital |
|
| 12,289,664 |
|
|
| 1,700,044 |
|

| Page 47 of 90 |
| Table of Contents |
The Company’s working capital balance increased substantially during the year ended August 31, 2021, due to the cash infusion from the sale of assets ($273,373), the net proceeds of an underwritten public offering ($9,471,497) and the exercise of warrants issued with the shares of the underwritten public offering ($4,015,043). The Company maintained a positive and strong working capital position throughout the year despite a healthy increase in expenditures, particularly in our R&D programs.
Cash Flows |
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
Cash flows (used in) provided by operating activities |
|
| (3,997,590 | ) |
|
| (2,628,450 | ) |
Cash flows (used in) provided by investing activities |
|
| 193,880 |
|
|
| (26,843 | ) |
Cash flows (used in) provided by financing activities |
|
| 13,427,758 |
|
|
| 2,663,895 |
|
Cash flows (used in) provided by discontinued operations |
|
| 3,000 |
|
|
| (34,816) |
|
Increase in cash |
|
| 9,624,048 |
|
|
| 8,602 |
|
Operating Activities
Net cash used in operating activities was $3,997,590 for the year ended August 31, 2021, compared with $2,628,450 during the same period in 2020. The increase in cash used in operating activities during fiscal 2021 was primarily driven by increased research and development programs and office and administrative expenditures, particularly on increased advertising and investor relations activities.
Investing Activities
Net cash provided by investing activities was $193,880 (2020 ($26,843)) for the year ended August 31, 2021, is due to the cash proceeds received on the sale of assets and further investment in our US patent portfolio.
Financing Activities
Net cash provided from financing activities was $13,427,758 during the year ended August 31, 2021, compared to $2,663,895 during the same period in 2020. During the year ended August 31, 2021, cash provided by financing activities was primarily driven by the issuance of common stock supplemented by the exercise of warrants related to underwritten public offering.
COVID-19
As the repercussions of COVID-19 reverberate around the world, the effects on Lexaria’s operations have been relatively minor. We have experienced some difficulty in recruiting R&D and administrative staff but as of the date of this report we have filled these positions and expect to accelerate our in-house research efforts throughout 2022. We have also experienced some delay in getting test results of our R&D programs due to supply-chain factors that could be attributed to the virus. Supply chain issues have also had some, but not significant, impact on securing ingredients for our B2B production. As the world re-opens, we will expect to increase spending on travel as we seek out commercial partners and further our advertising and investor relations efforts.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.

| Page 48 of 90 |
| Table of Contents |
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with the US GAAP. Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management's application of accounting policies.
We believe that understanding the basis and nature of the estimates and assumptions involved with the aspects of our financial statements are critical to an understanding of our financial statements as more particularly described in Note 2 to our audited annual consolidated financial statements included herein.
While our significant accounting policies are described in more detail in the notes to the consolidated financial statements appearing elsewhere in this report, we believe that the following accounting policies and estimates are those most critical to the preparation of our consolidated financial statements:
Stock-based compensation
We account for our stock-based compensation awards in accordance with the FASB ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees, including grants of employee stock options and modifications to existing agreements, to be recognized in the consolidated statements of operations and comprehensive loss based on their fair values. We use the Black-Scholes option-pricing model to determine the fair value of options granted.
Compensation expense related to our stock-based awards to employees, executives and directors have service-based vesting conditions and are recognized on a straight-line basis based on the grant date fair value over the associated service period of the award, generally the vesting term. The vesting terms of each grant is determined by the board of directors and typically have a 5-year contractual term.
The fair value estimation of options requires the input of subjective assumptions, including expected life of the option, stock price volatility, the risk-free interest rate, and expected dividends. The assumptions used in our Black-Scholes option-pricing model represent our best estimates involving numerous variables, uncertainties, assumptions, and the application judgment. They are inherently subjective. If any assumptions change, our stock-based compensation expense could be materially different in the future.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
As a “Smaller Reporting Company”, this Item and the related disclosure is not required.

| Page 49 of 90 |
| Table of Contents |
Item 8. Financial Statements and Supplementary Data
Report of Independent Registered Public Accounting Firm
To the Shareholders and Directors of
Lexaria Bioscience Corp.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Lexaria Bioscience Corp. (the “Company”) as of August 31, 2021 and 2020, and the related consolidated statements of operations and comprehensive loss, cash flows, and stockholders’ equity for each of the two years in the period ended August 31, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of August 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period ended August 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
We have not identified any critical audit matters for the years ended August 31, 2021 and 2020.
We have served as the Company’s auditor since 2016.
| /s/ DAVIDSON & COMPANY LLP | |||
| Vancouver, Canada | Chartered Professional Accountants | ||
|
| ||
| November 26, 2021 |


| Page 50 of 90 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP. | ||||||||
CONSOLIDATED BALANCE SHEET | ||||||||
(Expressed in U.S. Dollars) | ||||||||
| ||||||||
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
| ASSETS |
|
|
|
|
|
| ||
| Current |
|
|
|
|
|
| ||
| Cash |
| $ |
|
| $ |
| ||
| Marketable securities |
|
|
|
|
|
| ||
| Accounts receivable |
|
|
|
|
|
| ||
| Inventory |
|
|
|
|
|
| ||
| Prepaid expenses and deposit |
|
|
|
|
|
| ||
| Total Current Assets |
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
| Non-current assets, net |
|
|
|
|
|
|
|
|
| Right-of-use assets |
|
|
|
|
|
| ||
| Intellectual property |
|
|
|
|
|
| ||
| Property and equipment |
|
|
|
|
|
| ||
| Total Non-current Assets |
|
|
|
|
|
| ||
| TOTAL ASSETS |
| $ |
|
| $ |
| ||
|
|
|
|
|
|
|
|
|
| LIABILITIES |
|
|
|
|
|
|
|
|
| Current |
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
| $ |
|
| $ |
| ||
| Deferred revenue |
|
|
|
|
|
| ||
| Due to related party |
|
|
|
|
|
| ||
| Loan payable |
|
|
|
|
|
| ||
| Lease liabilities |
|
|
|
|
|
| ||
| Total Current Liabilities |
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
| Long Term |
|
|
|
|
|
|
|
|
| Lease liabilities - long term |
|
|
|
|
|
| ||
| Loan payable |
|
|
|
|
|
| ||
| Total Long Term Liabilities |
|
|
|
|
|
| ||
| TOTAL LIABILITIES |
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
| STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
| Share capital |
|
|
|
|
|
|
|
|
| Authorized: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Issued and outstanding: |
|
|
|
|
|
|
|
|
| and |
|
|
|
|
|
| ||
| Additional paid-in capital |
|
|
|
|
|
| ||
| Deficit |
|
| ( | ) |
|
| ( | ) |
| Equity attributable to shareholders of the Company |
|
|
|
|
|
| ||
| Non-Controlling Interest |
|
| ( | ) |
|
| ( | ) |
| Total Stockholders' Equity |
|
|
|
|
|
| ||
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
| $ |
|
| $ |
| ||
The accompanying notes are an integral party of these consolidated financial statements.

| Page 51 of 90 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP. | ||||||||
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS | ||||||||
(Expressed in U.S. Dollars except number of shares) | ||||||||
|
|
| ||||||
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
| Revenue |
| $ |
|
| $ |
| ||
| Cost of goods sold |
|
|
|
|
|
| ||
| Gross profit |
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
| Operating Expenses |
|
|
|
|
|
|
|
|
| Research and development |
|
|
|
|
|
| ||
| General and administrative |
|
|
|
|
|
| ||
| Total operating expenses |
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
| ( | ) |
|
| ( | ) |
|
|
|
|
|
|
|
|
|
| Gain on disposal of assets |
|
|
|
|
|
| ||
| Discontinued operations |
|
| ( | ) |
|
|
| |
|
|
|
|
|
|
|
|
|
| Net loss and comprehensive loss for the year |
| $ | ( | ) |
| $ | ( | ) |
| Net loss and comprehensive loss attributable to: |
|
|
|
|
|
|
|
|
| Common shareholders |
| $ | ( | ) |
| $ | ( | ) |
| Non-controlling interest |
| $ | ( | ) |
| $ | ( | ) |
|
|
|
|
|
|
|
|
|
| Basic and diluted loss per share |
| $ | ( | ) |
| $ | ( | ) |
Basic and diluted earnings (loss) per share from discontinued operations |
| $ | ( | ) |
| $ | |
|
|
|
|
|
|
|
|
|
|
| Weighted average number of common shares outstanding |
|
|
|
|
|
|
|
|
| - Basic and diluted |
|
|
|
|
|
| ||
The accompanying notes are an integral part of these consolidated financial statements.

| Page 52 of 90 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP. | ||||||||
CONSOLIDATED STATEMENT OF CASH FLOWS | ||||||||
(Expressed in U.S. Dollars) | ||||||||
|
|
| ||||||
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
| Cash flows used in operating activities |
|
|
|
|
|
| ||
| Net loss and comprehensive loss |
| $ | ( | ) |
| $ | ( | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
| Stock based compensation |
|
|
|
|
|
| ||
| Depreciation and amortization |
|
|
|
|
|
| ||
| Inventory write-off |
|
|
|
|
|
| ||
| Bad debt |
|
|
|
|
|
| ||
| Amortization on right of use asset |
|
|
|
| ||||
| Realized loss on disposal of marketable securities |
|
|
|
|
|
| ||
| Unrealized loss on marketable securities |
|
|
|
|
|
| ||
| Gain on asset disposal |
|
| ( | ) |
|
|
| |
| Common shares issued for services |
|
|
|
|
|
| ||
| Warrants issued for services |
|
|
|
|
|
| ||
Lease accretion |
|
|
|
|
|
| ||
| Change in working capital |
|
|
|
|
|
|
|
|
| Accounts receivable |
|
|
|
|
| ( | ) | |
| Inventory |
|
|
|
|
|
| ||
| Prepaid expenses and deposits |
|
| ( | ) |
|
| ( | ) |
| Accounts payable and accrued liabilities |
|
|
|
|
| ( | ) | |
| Due to related parties |
|
| ( | ) |
|
|
| |
| Deferred revenue |
|
| ( | ) |
|
|
| |
| Net cash used in operating activities |
| $ | ( | ) |
| $ | ( | ) |
|
|
|
|
|
|
|
|
|
| Cash flows from (used in) investing activities |
|
|
|
|
|
|
|
|
| Sale of marketable securities |
|
|
|
|
|
| ||
| Intellectual property |
|
| ( | ) |
|
| ( | ) |
| Asset disposition |
|
|
|
|
|
| ||
| Net cash from (used in) investing activities |
| $ |
|
| $ | ( | ) | |
|
|
|
|
|
|
|
|
|
| Cash flows from financing activities |
|
|
|
|
|
|
|
|
| Long term loan |
|
| ( | ) |
|
|
| |
Lease payments |
|
| ( | ) |
|
| ( | ) |
| Proceeds from issuance of equity |
|
|
|
|
|
| ||
| Proceeds from warrant exercises |
|
|
|
|
|
| ||
| Net cash from financing activities |
| $ |
|
| $ |
| ||
|
|
|
|
|
|
|
|
|
| Increase in cash |
|
|
|
|
|
| ||
| Cash, beginning of year |
|
|
|
|
|
| ||
| Cash, end of year |
| $ |
|
| $ |
| ||
|
|
|
|
|
|
|
|
|
| Supplemental information of cash flows: |
|
|
|
|
|
|
|
|
| Income taxes paid in cash |
| $ | ( | ) |
| $ | ( | ) |
| Marketable securities received on amounts receivable |
| $ |
|
| $ |
| ||
The accompanying notes are an integral part of these consolidated financial statements.

| Page 53 of 90 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(Expressed in U.S. Dollars except number of shares)
|
| SHARES |
|
| AMOUNT $ |
|
| ADDITIONAL PAID-IN CAPITAL $ |
|
| DEFICIT $ |
|
| NCI $ |
|
| TOTAL STOCKHOLDERS’ EQUITY $ |
| ||||||
Balance August 31, 2019 |
|
|
|
|
|
|
|
|
|
|
| ( | ) |
|
|
|
|
|
| |||||
Shares issued for services |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock based compensation |
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Warrants issued for services |
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Exercise of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Private Placements |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Net loss |
|
| - |
|
|
|
|
|
|
|
|
| ( | ) |
|
|
|
|
| (3,933,996 | ) | |||
Non-controlling interest |
|
| - |
|
|
|
|
|
|
|
|
|
|
|
| ( | ) |
|
| ( | ) | |||
Balance August 31, 2020 |
|
|
|
|
|
|
|
|
|
|
| ( | ) |
|
| ( | ) |
|
| 2,482,258 |
| |||
Shares issued for services |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock based compensation |
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Warrants issued for services |
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Exercise of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Private placement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Net loss |
|
| - |
|
|
|
|
|
|
|
|
| ( | ) |
|
|
|
|
| (4,027,006 | ) | |||
Non-controlling interest |
|
| - |
|
|
|
|
|
|
|
|
|
|
|
| ( | ) |
|
| ( | ) | |||
Balance August 31, 2021 |
|
|
|
|
|
|
|
|
|
|
| ( | ) |
|
| ( | ) |
|
| 13,063,552 |
| |||
The accompanying notes are an integral part of these consolidated financial statements.

| Page 54 of 90 |
| Table of Contents |
LEXARIA BIOSCIENCE CORP. NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS August 31, 2021 |
1. Nature of Business
Lexaria Bioscience Corp. (“Lexaria”, “we”, “our” or the “Company”) is a biotechnology company pursuing the enhancement of the bioavailability of a diverse and broad range of active pharmaceutical ingredients (“API”) using our proprietary DehydraTECH drug delivery technology.
Revenues are received from licensing the Company’s patented technology to partners who pay either a license fee to use DehydraTECH in the manufacturing of their own products or purchase DehydraTECH manufactured products made to their specifications by Lexaria. The Company has relationships with several consumer products companies in the CBD and nutraceuticals spaces that use Lexaria’s technology in consumer goods being sold online and at retailers in the US and Canada.
Going Concern Consideration
The Company’s consolidated financial statements included herein have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) and in accordance with accounting principles generally accepted in the United States (“US GAAP”) applicable to a going concern which assumes the Company will have sufficient funds to pay it operational, research and development and capital expenditures for a period of at least 12 months from the date this financial report.
Since inception, the Company has incurred significant operating and net losses. The losses attributable to common shareholders were $
On January 12, 2021, the Company closed an underwritten public offering for net proceeds of $
The Company has evaluated whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company's ability to continue as a going concern. As of August 31, 2021, the Company had cash and cash equivalents of approximately $

| Page 55 of 90 |
| Table of Contents |
Impacts of COVID-19 Pandemic
The emergence of the COVID-19 pandemic in 2020 continues to present uncertainty and unforecastable new risks to the Company and its business plans. As of August 31, 2021, there has been no material impact on the Company’s financial position as a direct result of the pandemic. However, the Company has experienced some supply chain disruptions and shortages in the timely procurement of ingredients and supplies used in both our R&D activities and production. Management views this situation as transitory but cannot predict the length of time it may take for these disruptions to dissipate or if there will be a significant economic effect on the Company’s operations. In the interim, it may cause delays in carrying out our research studies and in our production schedules.
Restrictions on international travel presents a challenge in carrying out normal business activities related to corporate finance efforts and the pursuit of new customers throughout North America who might otherwise access to our licensees’ retail products. As a result, the pandemic has increased the risk of lower revenues and higher losses.
During the year ended August 31, 2020, we were in receipt of C$
We continue to actively monitor the evolving effects of COVID-19 and may take further actions that alter our operations, including those that may be required by federal, state, provincial, or local authorities, or that we determine are in the best interests of our employees and other third parties with which we do business. We do not know when it will become practical to relax or eliminate some or all these measures entirely. The economic effect of a prolonged pandemic is difficult to predict and could result in material financial impact in the Company’s future reporting periods.
2. Significant Accounting Policies
a) Basis of presentation
These consolidated financial statements have been prepared in conformity with generally accepted accounting principles of the United States (“US GAAP”) and pursuant to the rules and regulations of the SEC. All amounts, unless otherwise stated, are in U.S. dollars.
On December 9, 2020, the Company completed the sale of the business assets in the THC related segment of our subsidiary Lexaria CanPharm ULC. As a result, the related financial results pertaining to the sale are reflected in our consolidated statement of operations, retrospectively, as discontinued operations beginning in the first quarter of fiscal 2021.
On January 11, 2021, the Company effected a 1-for-30 reverse stock split with no fractional shares issued. All share, option, warrant and per share information within these consolidated financial statements have been retroactively restated accordingly.

| Page 56 of 90 |
| Table of Contents |
b) Revenue recognition
Licensing revenue from intellectual property
We recognize revenue for license fees at a point in time following the transfer of our intellectual property, namely our patented lipid nutrient infusion technology DehydraTECH for infusing Active Pharmaceutical Ingredients (“API”), to the licensee, which occurs on delivery of documentation.
Usage fees from intellectual property
We recognize revenue for usage fees when usage of our DehydraTECH intellectual property occurs by licensees infusing an API into one or more of their product lines for sale.
Product revenue
Revenue from the sale of products is recognized when the sales price is fixed or determinable, there is persuasive evidence that an arrangement exists, delivery has occurred and collectability is reasonably assured.
c) Inventory and cost of sales
The Company’s inventory consists of raw materials, work in progress and finished goods. In all classes, inventory is valued at the lower of cost or market. Cost is determined on a first-in, first-out basis.
Cost of sales includes all expenditures incurred in bringing the goods to the point of sale. Inventory costs and costs of sales include direct costs of the raw material, inbound freight charges, warehousing costs, handling costs (purchasing and receiving) and overhead expenses.
d) Cash and cash equivalents
Cash and cash equivalents include cash-on-hand and demand deposits with financial institutions and other short-term investments with maturities of less than three months when acquired and convertible to known cash amounts. The Company had no cash equivalents as at August 31, 2021 or August 31, 2020.
e) Equipment
Equipment is stated at cost less accumulated depreciation and impairment and depreciated using the straight-line method over their useful lives of the various asset classes. Laboratory equipment, office furniture and computer equipment are depreciated over
f) Intellectual property
Capitalized patent costs represent legal costs incurred in pursuing patents applications in the United States. When such applications result in patents being issued, the directly related capital cost is amortized over the life of the patent on a straight-line basis.

| Page 57 of 90 |
| Table of Contents |
g) Stock-based compensation
The Company accounts for its stock-based compensation awards whereby all stock-based payments to employees, including grants of employee stock options, are recognized as expenses in the statements of operations based on the fair value at grant date. For stock options granted to employees, executives and to members of the Board of directors for their services, the Company estimates the grant date fair value of each option award using the Black-Scholes option-pricing model. The use of the Black-Scholes option-pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk-free interest rates and expected dividend yields of the common stock.
Stock-based payments issued to non-employees are recorded at their fair values and are periodically revalued as the equity instruments vest and are expensed over the related service period. The Company recognizes stock-based compensation expense on vesting for equity instruments granted.
h) Loss per share
The calculation of loss per share uses the weighted average number of shares outstanding during the year. Diluted net income per share includes the effect, if any, from the potential exercise or conversion of securities, such as restricted stock and stock options, which would result in the issuance of incremental shares of common stock. Diluted loss per share is equivalent to basic loss per share if the potential exercise of the equity-based financial instruments was anti-dilutive.
i) Foreign currency translation
The Company maintains its accounting records in US dollars. At the transaction date, each asset, liability, revenue, and expense that was acquired or incurred in a foreign currency is translated into US dollars by using the exchange rate in effect at that date; at the year end, monetary assets and liabilities are translated at the exchange rate in effect at that date. The resulting foreign exchange gains and losses are included in profit or loss.
j) Financial instruments
When measuring fair value, the Company seeks to maximize the use of observable inputs and minimize the use of unobservable inputs. This establishes a fair value hierarchy based on the level of independent objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. Inputs are prioritized into three levels used to measure fair value:
| · | Level 1 - Quoted prices in active markets for identical assets or liabilities. |
|
|
|
| · | Level 2 - Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable and |
|
|
|
| · | Level 3 - Unobservable inputs that are supported by little or no market activity, therefore requiring an entity to develop its own assumptions about the assumptions that market participants would use in pricing. |
The Company’s financial instruments consist primarily of cash, marketable securities, accounts receivable and payable, accrued liabilities, loan payable and due to related parties. The carrying amounts of cash, accounts receivable and payable, accrued liabilities, loan payable and due to related parties approximate their fair values due to their short maturities or quoted market prices.

| Page 58 of 90 |
| Table of Contents |
The Company’s headquarters and operations are located in Canada which results in exposure to market risks from fluctuations in foreign currency rates. The foreign currency exchange risk is the financial risk to the Company’s operations that arise from fluctuations in foreign exchange rates and the degree of volatility of these rates. Currently, the Company does not use derivative instruments to reduce its exposure to foreign currency risk as the impact of a change in a few basis points for USD/CAD is not expected to be material.
k) Income taxes
The Company recognizes deferred tax liabilities and assets for the expected future tax consequences of events that have been recognized in the Company’s financial statements or tax returns using the liability method. Under this method, deferred tax liabilities and assets are determined based on the temporary differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect in the year in which the differences are expected to reverse.
l) Impairment of long-lived assets
Long-lived assets, including equipment and intangible assets, such as the Company’s patents, are assessed for potential impairment when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. An impairment loss is recognized when the carrying amount of the long-lived asset is not recoverable and exceeds its fair value. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. Any required impairment loss is measured as the amount by which the carrying amount of the long-lived asset exceeds its fair value and is recorded as a reduction in the carrying value of the related asset and a charge to the profit or loss. Intangible assets with indefinite lives are tested for impairment annually and in interim periods if certain events occur indicating that the carrying value of the intangible assets may be impaired.
m) Comprehensive income
The Company discloses comprehensive income (loss), its components, and accumulated balances on its Statement of Stockholders’ Equity. Comprehensive income (loss) comprises equity changes except those transactions resulting from investments by stakeholders and owners and distributions to owners.
n) Credit risk and receivable concentration
The Company places its cash with a high credit quality financial institution. As of August 31, 2021, the Company had approximately $
Included in amounts relievable is $
In the year ended August 31, 2021, one licensee accounted for
As at August 31, 2021, we had $Nil (2020 – $
As at August 31, 2021, the Company had $

| Page 59 of 90 |
| Table of Contents |
o) Commitments and contingencies
The Company policy is to record accruals for any such loss contingencies when it is probable that a liability has been incurred and the amount of loss can be reasonably estimated. In the event that estimates or assumptions prove to differ from actual results, adjustments are made in subsequent periods to reflect more current information. Historically, the Company has not experienced any material claims.
The Company, from time to time, may be subject to legal claims and proceedings related to matters arising in the ordinary course of business. Management has no knowledge of any such claim against the Company with, at minimum, a reasonable possibility that a material loss may be incurred.
p) Research and development
Research and development costs are expensed as incurred. These expenditures are comprised of both in-house research programs including consultants and employee-related expenses and through third-party contracts including consultants, research organizations and contract manufacturing.
q) Leases
On September 1, 2019, we adopted ASC Topic 842, Leases (“ASC 842”) using the optional transition method and applied the standard only to leases that existed at that date. Under the optional transition method, we do not need to restate the comparative periods in transition and will continue to present financial information and disclosures for periods before September 1, 2019, in accordance with ASC Topic 840. We have elected the package of practical expedients allowed under ASC Topic 842, which permits us to account for our existing operating leases as operating leases under the new guidance, without reassessing our prior conclusions about lease identification, lease classification and initial direct cost. As a result of the adoption of the new lease accounting guidance on September 1, 2019, we recognized operating lease right-of-use assets of $
We determined the initial classification and measurement of our right-of-use assets and lease liabilities at the lease commencement date and thereafter if modified. The lease term includes any renewal options and termination options that we are reasonably certain to exercise. The present value of lease payments is determined by using the interest rate implicit in the lease, if that rate is readily determinable; otherwise, we use our incremental borrowing rate. The incremental borrowing rate is determined by using the rate of interest that we would pay to borrow on a collateralized basis an amount equal to the lease payments for a similar term and in a similar economic environment.
Rent expense for operating leases is recognized on a straight-line basis, unless the right-of-use asset has been impaired, over the reasonably certain lease term based on the total lease payments and is included in operating expenses in the consolidated statements of operations and comprehensive loss.
For operating leases that reflect impairment, we will recognize the amortization of the right-of-use asset on a straight-lined basis over the remaining lease term with rent expense still included in operating expenses in the consolidated statements of operations and comprehensive loss.
For all leases, rent payments that are based on a fixed index or rate at the lease commencement date are included in the measurement of lease assets and lease liabilities at the lease commencement date.

| Page 60 of 90 |
| Table of Contents |
We have elected the practical expedient to not separate lease and non-lease components. Our non-lease components are primarily related to property taxes and maintenance, which vary based on future outcomes, and thus differences to original estimates are recognized in rent expense when incurred.
3. Basis of Consolidation
These consolidated financial statements include the financial statements of the Company and its wholly owned subsidiaries; Lexaria Pharmaceutical Corp., Lexaria Hemp Corp., Lexaria CanPharm ULC, PoViva Corp., and Kelowna Management Services Corp. The Company owns
4. Estimates and Judgments
The preparation of financial statements in conformity with US GAAP requires us to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting year. Some of the Company’s accounting policies require us to make subjective judgments, often as a result of the need to make estimates of matters that are inherently uncertain. These accounting policies involve critical accounting estimates because they are particularly dependent on estimates and assumptions made by management about matters that are highly uncertain at the time the accounting estimates are made. Although we have used our best estimates based on facts and circumstances available to us at the time, different estimates reasonably could have been used. Changes in the accounting estimates used by the Company are reasonably likely to occur from time to time, which may have a material effect on the presentation of financial condition and results of operations.
The Company reviews these estimates, judgments, and assumptions periodically and reflect the effects of revisions in the period in which they are deemed to be necessary. We believe that these estimates are reasonable. However, actual results could differ from these estimates. Significant accounting estimates and assumptions are used for, but not limited to:
a) The Valuation of Deferred Tax Assets
Judgment is required in determining whether deferred tax assets are recognized on the balance sheet. The recognition of deferred tax assets requires management to assess the likelihood that the Company will generate taxable income in future periods to utilize the deferred tax assets. Due to the Company’s history of losses, deferred tax assets have not been recognized by Lexaria.
b) Value of Stock Options and Warrants
The Company provides compensation benefits to its employees, directors, officers, and consultants, through a stock option plan. The fair value of each option award is estimated on the date of grant using the Black-Scholes option-pricing model. Expected volatility assumptions used in the model are based on the historical volatility of the Company’s share price. The Company uses historical data to estimate the period of option exercises for use in the valuation model. The risk-free interest rate for the expected term of the option is based on the yields of government bonds. Changes in these assumptions, especially the share price volatility and the expected life determination could have a material impact on the Company’s profit and loss for the years presented. All estimates used in the model are based on historical data which may not be representative of future results.

| Page 61 of 90 |
| Table of Contents |
c) Disposals of Assets - Value of Note Receivable
The Asset Purchase Agreement for the sale of assets to Hill Street Beverages included C$2m note receivable as partial payment of the agreement. The Note does not contain a fixed repayment schedule nor a maturity date. The repayment of the Note is based on the purchaser repaying the outstanding value of the Note and interest from the future revenues generated from an untested market with no existing revenue streams. Therefore, with any repayment being highly doubtful, management determined at that time that the value of the note to be notional and recorded the note at a $NIL value for accounting purposes. Any subsequent payment of principle and/or interest is to be recorded in the period received as income.
5. Recent Accounting Guidance
Pronouncements Issued but Not Yet Adopted
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The FASB subsequently issued amendments to ASU 2016-13, which have the same effective date and transition date of January 1, 2023. These standards require that credit losses be reported using an expected losses model rather than the incurred losses model that is currently used, and establishes additional disclosures related to credit risks. For available-for-sale debt securities with unrealized losses, these standards now require allowances to be recorded instead of reducing the amortized cost of the investment. These standards limit the amount of credit losses to be recognized for available-for-sale debt securities to the amount by which carrying value exceeds fair value and requires the reversal of previously recognized credit losses if fair value increases. The Company does not currently expect the adoption of these standards to have a material impact on its consolidated financial statements.
6. Accounts and Other Receivables
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
Trade and deposits receivable |
|
|
|
|
|
| ||
Territory license fee receivable |
|
|
|
|
|
| ||
Sale of assets - shares receivable |
|
|
|
|
|
| ||
Sales tax receivable |
|
|
|
|
|
| ||
|
|
|
|
|
|
| ||

| Page 62 of 90 |
| Table of Contents |
7. Inventory
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
Raw materials |
|
|
|
|
|
| ||
Work in progress |
|
|
|
|
|
| ||
Finished goods |
|
|
|
|
|
| ||
|
|
|
|
|
|
| ||
During the year ended August 31, 2021, the Company divested its operations in on-line sales of consumer products and as a result finished goods inventory valued at $
8. Intellectual Property
The following is a list of capitalized US patents held by the Company.
Issued Patent # | Patent Issuance Date | Patent Family |
Food and Beverage Compositions Infused With Lipophilic Active Agents and Methods of Use Thereof | ||
|
Schedule of continuity for capitalized patents:
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
Balance – Beginning |
|
|
|
|
|
| ||
Addition |
|
|
|
|
|
| ||
Amortization |
|
| ( | ) |
|
| ( | ) |
Balance – Ending |
|
|
|
|
|
| ||
Patents are amortized over their legal life of 20 years. |
|
|
|
|
|
|
|
|

| Page 63 of 90 |
| Table of Contents |
9. Property & Equipment
| Year Ended August 31, 2021 |
| Cost |
|
| Amortization |
|
| Disposals |
|
| Accumulated Amortization |
|
| Net Balance |
| |||||
|
| $ |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| |||||
Leasehold improvements |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) |
|
|
| |||
Computers |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) |
|
|
| |||
Furniture fixtures equipment |
|
|
|
|
| ( | ) |
|
| ( | ) |
|
| ( | ) |
|
|
| ||
Lab equipment |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) |
|
|
| |||
|
|
|
|
|
| ( | ) |
|
| ( | ) |
|
| ( | ) |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended August 31, 2020 |
| Cost |
|
| Amortization |
|
| Disposals |
|
| Accumulated Amortization |
|
| Net Balance |
| |||||
|
|
| $ |
|
|
| $ |
|
|
| $ |
|
|
| $ |
|
|
| $ |
|
Leasehold improvements |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) |
|
|
| |||
Computers |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) |
|
|
| |||
Furniture fixtures equipment |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) |
|
|
| |||
Lab equipment |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) |
|
|
| |||
|
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) |
|
|
| |||
During the year ended August 31, 2021, $
10. Accounts Payable and Accrued Liabilities
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
Accounts Payable |
|
|
|
|
|
| ||
Vendors payable |
|
|
|
|
|
| ||
| Accrued Liabilities |
|
|
|
|
|
|
|
|
| Corporate tax payable |
|
|
|
|
|
| ||
| Vendors payable |
|
|
|
|
|
| ||
| Total |
|
|
|
|
|
| ||
11. Common Shares and Warrants
Fiscal 2021 Activity
On January 11, 2021, the Company filed an amendment and restatement of its articles of incorporation to effectuate a

| Page 64 of 90 |
| Table of Contents |
During the year ended August 31, 2021, the Company closed an underwritten public offering for an aggregate total of
During the year ended August 31, 2021, the Company issued
During the year ended August 31, 2021, the Company granted
A summary of share issuances for the year ended August 31, 2021, is presented below:
| Type of Issuance |
| Number of Shares |
|
| Total Value $ |
| ||
Warrant exercise |
|
|
|
|
|
| ||
Private placement (1) |
|
|
|
|
|
| ||
Per agreements(2) |
|
|
|
|
|
| ||
|
|
|
|
|
|
| ||
(1) Fees of $
(2) The Company awarded restricted common shares as required by consulting contracts.
Fiscal 2020 Activity
During the year ended August 31, 2020, the Company closed two tranches of a non-brokered private placement for an aggregate total of
The Company also issued an aggregate of

| Page 65 of 90 |
| Table of Contents |
Presented below is a summary of options exercised, share issuance and as per agreement requirements for the year ended August 31, 2020:
| Type of Issuance |
| Number of Shares |
|
| Total Value $ |
| ||
Warrants exercised |
|
| - |
|
|
| - |
|
Options exercised |
|
| 7,333 |
|
|
|
| |
Private placement (1) |
|
|
|
|
|
| ||
Per agreements(2) |
|
|
|
|
|
| ||
|
|
|
|
|
|
| ||
(1) Total fees of $
(2) The Company awarded the restricted common shares as required by consulting contracts.
In the year ended August 31, 2020, the Company granted a total of
Presented below is a continuity schedule for warrants:
|
| Number of Warrants |
|
| Weighted Average Exercise Price $ |
| ||
Balance August 31, 2019 |
|
|
|
|
|
| ||
Cancelled/Expired |
|
| ( | ) |
|
|
| |
Issued |
|
|
|
|
|
| ||
Balance August 31, 2020 |
|
|
|
|
|
| ||
Cancelled/Expired |
|
| ( | ) |
|
|
| |
Exercised |
|
| ( | ) |
|
|
| |
Issued |
|
|
|
|
|
| ||
Balance August 31, 2021 |
|
|
|
|
|
| ||
The fair value of share purchase warrants granted as compensation units, and compensatory warrants, was estimated as of the date of the grant by using the Black-Scholes option pricing model with the following assumptions:
|
| August 31 2021 |
|
| August 31 2020 |
| ||
Expected volatility |
|
|
|
| ||||
Risk-free interest rate |
|
|
|
| ||||
Expected life |
|
|
|
| ||||
Dividend yield |
|
| % |
|
| % | ||
Estimated fair value per warrant |
| $ |
|
| $ |
| ||

| Page 66 of 90 |
| Table of Contents |
Presented below is a summary of warrants outstanding as of August 31, 2021:
Number of Warrants |
|
| Weighted Average Remaining Contractual Life |
| Weighted Average Exercise Price $ |
| ||
|
|
|
|
|
| |||
|
|
|
|
|
| |||
|
|
|
|
|
| |||
|
|
|
|
|
| |||
|
|
|
|
|
| |||
|
|
|
|
|
| |||
|
|
|
|
|
| |||
|
|
|
|
|
| |||
12. Stock Options
The Company established an Equity Incentive Plan whereby the board of directors may, from time to time, grant up to
Stock options granted must be exercised no later than five years from the date of grant or such lesser period as determined by the Company’s Board of directors. The exercise price of an option is equal to or greater than the closing market price of the Company’s common shares on the day preceding the date of grant. The vesting terms of each grant are set by the Board of directors. The Company estimates the fair value of each stock option award on the measurement date using a Black-Scholes option pricing model.
During the year ended August 31, 2021, the Company cancelled its 2014 Stock Option Plan. All outstanding options expired during the year. During the year ending August 31, 2020, the 2007 Equity Incentive Plan and the 2010 Stock Option Plan were cancelled. Any outstanding options were cancelled and reissued under the Equity Incentive Plan.
Fiscal 2021 Activity
The Company granted the following stock options in the year ending August 31, 2021:
Quantity |
|
| Exercise Price $ |
|
| Life (Years) |
| |||
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
| |||
During the year,

| Page 67 of 90 |
| Table of Contents |
Fiscal 2020 Activity
The Company granted the following stock options in the year ending August 31, 2020:
Quantity |
|
| Exercise Price $ |
|
| Life (Years) |
| |||
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
| |||
| (1) |
|
|
|
|
|
| |||
(1) 132,067 vested, and 29,533 are subject to vesting provisions.
A continuity schedule for stock options is presented below:
|
| Options |
|
| Weighted Average Exercise Price $ |
|
| Weighted Average Remaining Contractual Term (Years) |
|
| Aggregate Intrinsic Value $ |
| ||||
Balance August 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Expired/Cancelled |
|
| ( | ) |
|
|
|
|
|
|
|
|
| |||
Exercised |
|
| ( | ) |
|
|
|
|
|
|
|
|
| |||
Granted |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Balance August 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Expired/Cancelled |
|
| ( | ) |
|
|
|
|
|
|
|
|
| |||
Granted |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Balance August 31, 2021 (Outstanding) |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Balance August 31, 2021 (Exercisable) |
|
|
|
|
|
|
|
|
|
|
|
| ||||
The intrinsic value of stock option awards that vested during the fiscal year represents the value of the Company’s closing stock price on the last trading day of the fiscal year in excess of the exercise price multiplied by the number of options that vested.
The fair value of options granted was estimated as of the date of the grant by using the Black-Scholes option pricing model with the following assumptions:
|
| August 31 2021 |
|
| August 31 2020 |
| ||
Expected volatility |
|
|
|
| ||||
Risk-free interest rate |
|
|
|
| ||||
Expected life |
| 5 years |
|
| 5 years |
| ||
Dividend yield |
|
| % |
|
| % | ||
Estimated fair value per option |
| $ |
|
| $ |
| ||

| Page 68 of 90 |
| Table of Contents |
13. Revenues
|
| August 31 2021 $ |
|
| August 31 2020 $ |
| ||
B2B sales |
|
|
|
|
|
| ||
Licensing revenue |
|
|
|
|
|
| ||
Other revenue |
|
|
|
|
|
| ||
|
|
|
|
|
|
| ||
The licensing fees consist of IP licensing fees for transfer of the DehydraTECH technology with the signing of definitive agreements and usage fees. The licensing fees include payments due upon transfer of the technology and installment payments that are receivable within 12 months.
The Company recognized $
14. Related Party Transactions
Due to related parties:
As at August 31, 2021, $5,233 (August 31, 2020 - $
15. Segment Information
The Company’s operations involve the development and usage, including licensing, of DehydraTECH. Lexaria is centrally managed and its chief operating decision makers, being the President and the CEO, use the consolidated and other financial information supplemented by revenue information by category of business-to-business product production and technology licensing to make operational decisions and to assess the performance of the Company. The Company has identified two reportable segments: Intellectual Property Licensing and B2B Production. Licensing revenues are significantly concentrated on three licensees.
For year ended August 31, 2021 |
| IP Licensing |
|
| B2B Product |
|
| Corporate |
|
| Consolidated Total |
| ||||
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
| External revenue |
|
|
|
|
|
|
|
|
|
|
|
| ||||
| Cost of goods sold |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) | ||
| Operating expenses |
|
| (1,864,527 | ) |
|
| (1,325,809 | ) |
|
| ( | ) |
|
| ( | ) |
| Segment loss |
|
| ( | ) |
|
| ( | ) |
|
| ( | ) |
|
| ( | ) |
| Total assets |
|
|
|
|
|
|
|
|
|
|
|
| ||||
For year ended August 31, 2020 |
| IP Licensing |
|
| B2B Product |
|
| Corporate |
|
| Consolidated Total |
| ||||
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
External revenue |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Cost of goods sold |
|
|
|
|
| ( | ) |
|
|
|
|
| ( | ) | ||
Operating expenses |
|
| ( | ) |
|
| ( | ) |
|
| ( | ) |
|
| ( | ) |
Segment loss |
|
| ( | ) |
|
| ( | ) |
|
| ( | ) |
|
| ( | ) |
Total assets |
|
|
|
|
|
|
|
|
|
|
|
| ||||

| Page 69 of 90 |
| Table of Contents |
Capital Asset by Region |
| Cost |
|
| Disposal US |
|
| Net Balance |
|
| Cost |
|
| Net Balance Canada |
|
| Net Balance Total |
| ||||||
Year Ended August 31, 2021 |
| $ |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||||
Leasehold Improvements |
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Computers |
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Furniture Fixtures Equipment |
|
|
|
|
| ) |
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Lab Equipment |
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
| ( | ) |
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended August 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Leasehold Improvements |
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Computers |
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Furniture Fixtures Equipment |
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Lab Equipment |
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
16. Commitments, Significant Contracts and Contingencies
Right of Use Assets - Operating Lease
Corporate offices and R&D lab space is leased in Kelowna, British Columbia, Canada until November 15, 2023, with an optional five-year extension. In addition to minimum lease payments, the lease requires us to pay property taxes and operating costs which are subject to annual adjustments.
|
| August 31, 2021 |
|
| August 31, 2020 |
| ||
|
| $ |
|
| $ |
| ||
Right of use assets - operating leases: |
|
|
|
|
|
| ||
| Amortization |
|
| ( | ) |
|
| ( | ) |
Total lease assets |
|
|
|
|
|
| ||
Liabilities: |
|
|
|
|
|
| ||
| Lease payments |
|
| ( | ) |
|
| ( | ) |
Interest accretion |
|
|
|
|
|
| ||
Total lease liabilities |
|
|
|
|
|
| ||
Operating lease cost |
| $ |
|
| $ |
| ||
Operating cash flows for lease |
| $ |
|
| $ |
| ||
Remaining lease term |
|
|
|
| ||||
Discount rate |
|
| % |
|
| % |

| Page 70 of 90 |
| Table of Contents |
Pursuant to the terms of the Company’s lease agreements in effect at August 31, 2021, the following table summarizes the Company’s maturities of operating lease liabilities:
|
| $ |
| |
2022 |
|
|
| |
2023 |
|
|
| |
2024 |
|
|
| |
Thereafter |
|
|
| |
Total lease payments |
|
|
| |
Less: imputed interest |
|
| ( | ) |
Present value of operating lease liabilities |
|
|
| |
Less: current obligations under leases |
|
| ( | ) |
Total |
|
|
| |
17. Prepaid Expenses
Prepaid expenses consist of the following as at August 31, 2021 and August 31, 2020:
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
| Advertising and conferences |
|
|
|
|
|
| ||
| Consulting |
|
|
|
|
| - |
| |
| Legal fees |
|
|
|
|
|
| ||
| Licence, filing fees, dues |
|
|
|
|
|
| ||
| Office and insurance |
|
|
|
|
|
| ||
| Research and development |
|
|
|
|
|
| ||
|
|
|
|
|
|
| ||
18. Marketable Securities
The components of Marketable Securities were as follows:
|
| Cost Basis |
|
| Unrealized Gains |
|
| Unrealized Losses |
|
| Total |
| ||||
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
August 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Common Stock |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Total |
|
|
|
|
|
|
|
| ( | ) |
|
|
| |||
August 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
|
|
|
|
|
|
| ( | ) |
|
|
|
| ||
Total |
|
|
|
|
|
|
|
| ( | ) |
|
|
| |||
Unrealized losses from common stock are due to market price movements. In Managements’ opinion based on the evaluation of available information at the year ended August 31, 2021, unrealized losses represent temporary impairments.

| Page 71 of 90 |
| Table of Contents |
19. Discontinued Operations
On November 19, 2020, the Company entered a definitive asset sale agreement through its wholly owned subsidiary Lexaria CanPharm ULC to sell certain assets for gross proceeds of C$
The sale closed on December 10, 2020, with the Company receiving C$
The Company received the second tranche of shares on August 9, 2021, as per the sale agreement. Based on the agreed terms, the value of the
The gain on the transaction is presented below:
| Gain on asset disposal |
| $ |
| |
| Book value of assets sold |
|
|
| |
| Cash consideration |
|
|
| |
| Shares received |
|
|
| |
| Shares receivable |
|
|
| |
| Promissory note |
|
|
| |
|
|
|
| |
The financial results of the group of assets sold are presented as income (loss) from discontinued operations, net of income taxes in our consolidated statement of income. The following table presents financial results of the assets:
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
| Revenue |
|
|
|
|
|
| ||
| Operating expenses |
|
| ( | ) |
|
| ( | ) |
| Net income (loss) |
|
| ( | ) |
|
|
| |

| Page 72 of 90 |
| Table of Contents |
The following table presents cash flows of discontinued operations:
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
| Cash flows used in discontinued operating activities |
|
|
|
|
|
| ||
| Net income |
|
| ( | ) |
|
|
| |
| Change in working capital |
|
|
|
|
| ( | ) | |
| Net cash provided by (used in) discontinued operating activities |
|
|
|
|
| ( | ) | |
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) discontinued operations |
|
|
|
|
| ( | ) | |
The following table presents the aggregate carrying amounts of the classes of assets and liabilities of discontinued operations of the assets:
|
| August 31 |
|
| August 31 |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
| $ |
|
| $ |
| ||
| Current Assets |
|
|
|
|
|
| ||
| Accounts receivable |
|
|
|
|
|
| ||
| Current Liabilities |
|
|
|
|
|
|
|
|
| Accounts payable |
|
|
|
|
|
| ||
20. Income Tax
The following table reconciles the income tax benefit at the U.S. Federal statutory rate to income tax benefit at the Company’s effective tax rates as at August 31, 2021 and 2020:
|
| August 31 2021 |
|
| August 31 2020 |
| ||
|
| $ |
|
| $ |
| ||
Loss before taxes |
|
| ( | ) |
|
| ( | ) |
Expected income tax recovery |
|
| ( | ) |
|
| ( | ) |
Non-deductible items |
|
| ( | ) |
|
|
| |
Change in estimates |
|
| ( | ) |
|
|
| |
Effect of changes in foreign and long-term tax rates |
|
|
|
|
|
| ||
Change in valuation allowance |
|
|
|
|
|
| ||
Total income taxes |
|
|
|
|
|
| ||

| Page 73 of 90 |
| Table of Contents |
Deferred taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes. Deferred tax assets at August 31, 2021 and 2020 are comprised of the following:
|
| August 31 2021 $ |
|
| August 31 2020 $ |
| ||
Non-capital losses |
|
|
|
|
|
| ||
Marketable securities |
|
| 14,270 |
|
|
| ||
Total unrecognized deferred tax assets |
|
|
|
|
|
| ||
The Company has net operating loss carry-forwards of approximately $
Year |
| Amount |
|
| Canada |
| ||
2026 |
|
|
|
|
| - |
| |
2025 |
|
|
|
|
| - |
| |
2026 |
|
|
|
|
| - |
| |
2027 |
|
|
|
|
| - |
| |
2028 |
|
|
|
|
| - |
| |
2029 |
|
|
|
|
| - |
| |
2030 |
|
|
|
|
| - |
| |
2031 |
|
|
|
|
| - |
| |
2032 |
|
|
|
|
| - |
| |
2033 |
|
|
|
|
| - |
| |
2034 |
|
|
|
|
| - |
| |
2035 |
|
|
|
|
| - |
| |
2036 |
|
|
|
|
| - |
| |
2037 |
|
|
|
|
| - |
| |
2038 |
|
|
|
|
| - |
| |
2039 |
|
|
|
|
|
| ||
2040 |
|
|
|
|
| |||
Indefinite |
|
|
|
|
| - |
| |
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
| |||
21. Subsequent Events
Subsequent to the year ended August 31, 2021,

| Page 74 of 90 |
| Table of Contents |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
There were no disagreements related to accounting principles or practices, financial statement disclosure, internal controls or auditing scope or procedure during the two fiscal years and their respective interim periods.
Item 9A. Controls and Procedures
Management’s Report on Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer (also our Principal Executive Officer) and our Chief Financial Officer (also our Principal Financial and Accounting Officer) to allow for timely decisions regarding required disclosure.
As of August 31, 2021, the end of our fiscal year covered by this report, we carried out an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer (also our Principal Executive and Financial Reporting and Accounting Officers), of the effectiveness of the design and operation of our disclosure controls and procedures. Based on the foregoing, our Chief Executive Officer and the Chief Financial Officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Responsibility estimates and judgments by management are required to assess the expected benefits and related costs of control procedures. The objectives of internal control include providing management with reasonable, but not absolute, assurance that assets are safeguarded against loss from unauthorized use or disposition, and that transactions are executed in accordance with management’s authorization and recorded properly to permit the preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States. Our management assessed the effectiveness of our internal control over financial reporting as of August 31, 2021. In making this assessment, our management used the criteria set forth in the report entitled “Internal Control — Integrated Framework” published by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Our management has concluded that, as of August 31, 2021, our internal control over financial reporting is effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with US generally accepted accounting principles. Our management reviewed the results of their assessment with our Board of directors.
Inherent Limitations on Effectiveness of Controls
Internal control over financial reporting has inherent limitations which include but is not limited to the use of independent professionals for advice and guidance, interpretation of existing and/or changing rules and principles, segregation of management duties, scale of organization, and personnel factors. Internal control over financial reporting is a process which involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements on a timely basis, however these inherent limitations are known features of the financial reporting process and it is possible to design into the process safeguards to reduce, though not eliminate, this risk. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.

| Page 75 of 90 |
| Table of Contents |
Changes in Internal Control over Financial Reporting
The fundamental controls and control processes remained consistent with prior years during the year ended August 31, 2021. In April 2021, the former CFO Mr. Allan Spissinger was replaced by the former controller, Mr. Greg Downey which required some of our controls and controls processes to be temporarily revised and updated based on personnel changes within the Company. There have been no changes in our internal controls over financial reporting that occurred during the year ended August 31, 2021, that have materially or are reasonably likely to materially affect our internal controls over financial reporting.
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
All directors of our Company hold office until the next annual meeting of the security holders or until their successors have been elected and qualified. The officers of our Company are appointed by our board of directors and hold office until their death, resignation, or removal from office. Our directors and executive officers, their ages, positions held, and duration as such, are as follows:
Name |
| Position Held with our Company |
| Age |
|
| Date First Elected Or Appointed |
| Date of Resignation |
| ||
Christopher Bunka |
| Chairman, Chief Executive Officer, and Director |
|
| 60 |
|
| Oct. 26, 2006 |
|
| - |
|
|
|
|
|
|
|
|
| Feb. 14, 2007 |
|
|
|
|
John Docherty |
| President and Director |
|
| 51 |
|
| Apr. 15, 2015 |
|
| - |
|
|
|
|
|
|
|
|
| Apr. 29, 2016 |
|
|
|
|
Gregory Downey |
| Chief Financial Officer |
|
| 61 |
|
| Apr. 15, 2021 |
|
| - |
|
Nicholas Baxter |
| Director |
|
| 67 |
|
| Jul. 8, 2011 |
|
| - |
|
Ted McKechnie |
| Director |
|
| 73 |
|
| Sept. 16, 2015 |
|
| - |
|
Al Reese, Jr. |
| Director |
|
| 71 |
|
| Jan 14, 2021 |
|
| - |
|
Allan Spissinger |
| Former Chief Financial Officer |
|
| 51 |
|
| Jun. 1, 2017 |
| Apr. 15, 2021 |
| |
Brian Quigley |
| Former Director |
|
| 47 |
|
| Aug. 14, 2019 |
| Jun. 21, 2021 |
| |
Business Experience
The following is a brief account of the business and education experience of each current director and executive officer during the past five years, indicating each person's principal occupation during the period.
Mr. Christopher Bunka – Chairman, Chief Executive Officer and Director
Mr. Bunka has been Chairman of the Board and CEO since 2006 and was primarily responsible for the corporate pivot from older business activities to bioscience. Mr. Bunka is a serial entrepreneur and has been involved in several private and public companies since the late 1980’s. He was well known for more than a decade as a part-time business commentator in print and radio, as well as an author. He has extensive experience in the capital markets, corporate governance, project acquisition and corporate finance. He is a named inventor on some of Lexaria’s pending patents.

| Page 76 of 90 |
| Table of Contents |
Since 1988, Mr. Bunka has been the CEO of CAB Financial Services Ltd., a private holding company located in Kelowna, Canada. He is a venture capitalist and corporate consultant.
Mr. John Docherty – President and Director
Mr. Docherty was appointed President of Lexaria effective April 15, 2015. Prior to Lexaria Mr. Docherty was former President and Chief Operating Officer of Helix BioPharma Corp. (TSX: HBP), where he led the company’s pharmaceutical development programs for its plant and recombinantly derived therapeutic protein product candidates.
Mr. Docherty is a senior operations and management executive with over 20 years experience in the pharmaceutical and biopharmaceutical sectors. He has worked with large multinational companies and emerging, private and publicly held start-ups. At Helix, Mr. Docherty was also instrumental in the areas of investor/stakeholder relations, capital raising, capital markets development, strategic partnering, regulatory authority interactions and media relations, and he also served as a management member of its board of directors. Prior to this, Mr. Docherty was President and a board member of PharmaDerm Laboratories Ltd., a Canadian drug delivery company that developed unique microencapsulation formulation technologies for use with a range of active compounds.
Mr. Docherty has also held positions with companies such as Astra Pharma Inc., Nu-Pharm Inc. and PriceWaterhouseCoopers’ former global pharmaceutical industry consulting practice. He is a named inventor on issued and pending patents and he has a M.Sc. in pharmacology and a B.Sc. in Toxicology from the University of Toronto.
He has served as a director of Lexaria since April 29, 2016.
Mr. Gregory Downey – Chief Financial Officer
Mr. Downey joined the Company in January 2019 as Controller and accepted the position of Chief Financial Officer in April 2021. Mr. Downey brings over 35 years of diverse financial experience in the mining, oil and gas, manufacturing, construction, and public sectors as well as providing business advisory and financial accounting services to many mid-sized organizations. In addition, Mr. Downey has a wide range of executive corporate experience having acted as the Chief Financial Officer and director of various public companies.
Mr. Downey obtained his Certified Management Accountant (CMA) designation in 1992 and is a member of the Chartered Professional Accountants (CPA) of British Columbia. He holds a diploma in Business Administration from the Southern Alberta Institute of Technology.
Mr. Nicholas Baxter - Director
Mr. Baxter was appointed as a member on the board of directors of Lexaria Corp. in 2009. Mr. Baxter received a Bachelor of Science (Honours) from the University of Liverpool in 1975, and has worked on oil & gas projects in many areas of the world. Since the 1980’s, he has worked with companies in the public markets both in the U.K. and in Canada. Mr. Baxter brings extensive real-world experience as a board member.

| Page 77 of 90 |
| Table of Contents |
Mr. Ted McKechnie – Director
Mr. McKechnie is a well-recognized thought leader in the Canadian food industry. In the past, Mr. McKechnie was president of Maple Leaf Foods, an owner and senior executive at Humpty Dumpty Snack Foods and a senior leader at Pepsi Co. After a distinguished career as an executive and marketer specializing in food manufacturing, he now focuses on moving the Canadian food sector into the future. Aside from being the chairman of Food Starter’s board, Mr. McKechnie is also the Chairman/CEO of The Davies Group and William Davies Consulting Inc. Mr. McKechnie is also a chairman of the board for Advanced Technology For Food Manufacturing, serves on the Board Of Governors for St Jerome’s University. Mr. McKechnie is often called upon by think tanks, the government and industry leaders to offer insights on how to grow the food sector and add more value to the Canadian economy.
Mr. Al Reese Jr. - Director
Mr. Reese has over 40 years experience in public and private businesses including as CFO of a formerly Nasdaq-listed energy company where he arranged finance transactions totaling over $10 billion dollars during his 20-year tenure. Mr. Reese was a Director and Chairman of the Audit Committee of a community bank in Texas for ten years until such time as it was acquired by a larger banking group in 2018 and currently serves as an Independent Director and Chairman of the Audit Committee for a privately held insurance company headquartered in The Woodlands, Texas. He has directed over 50 acquisitions and financings from as small as a few hundred thousand dollars to multibillion dollar transactions in both the domestic and international arenas. He has directed or participated in numerous due diligence examinations, both domestic and foreign and has held the responsibility for integrating the finance, accounting and managerial practices for acquisitions and dispositions in both domestic and foreign operations in both public and private companies.
Mr. Reese is a Certified Public Accountant (1974), and received his Bachelor of Business Administration degree from Texas A&M University in 1971, and his MBA from University of Houston in 1977.
Family Relationships
There are no family relationships among any of our officers or directors.
Involvement in Certain Legal Proceedings
None of our directors, executive officers, promoters, or control persons has been involved in any of the following events during the past five years:
1) | A petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing. |
|
|
2) | A conviction in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses). |
|
|
3) | The subject of any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities: |

| Page 78 of 90 |
| Table of Contents |
| i. | Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity, or |
|
|
|
| ii. | Engaging in any type of business practice; or |
|
|
|
| iii. | Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws. |
4) | The subject of any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in paragraph (f)(3)(i) of this section, or to be associated with persons engaged in any such activity. |
|
|
5) | Found by a court of competent jurisdiction in a civil action or by the SEC to have violated any Federal or State securities law, and the judgment in such civil action or finding by the SEC has not been subsequently reversed, suspended, or vacated. |
|
|
6) | Found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended, or vacated. |
|
|
7) | The subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended, or vacated, relating to an alleged violation of: |
| i. | Any Federal or State securities or commodities law or regulation; or |
|
|
|
| ii. | Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or |
|
|
|
| iii. | Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity. |
8) | The subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Delinquent Section 16(a) Reports
Section 16(a) of the Securities Exchange Act of 1934 requires our executive officers and directors and persons who own more than 10% of our common stock to file with the Securities and Exchange Commission initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our common stock and other equity securities, on Forms 3, 4 and 5 respectively. Executive officers, directors and greater than 10% shareholders are required by the SEC regulations to furnish us with copies of all Section 16(a) reports that they file.

| Page 79 of 90 |
| Table of Contents |
Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that during fiscal year ended August 31, 2021, all filing requirements applicable to our officers, directors, and beneficial owners of greater than 10% percent were complied with.
Code of Ethics
We adopted a Code of Ethics applicable to our senior financial officers and certain other finance executives, which is a "code of ethics" as defined by applicable rules of the SEC. Our Code of Ethics is attached as an exhibit to our Form SB-2 filed on September 20, 2007. If we make any amendments to our Code of Ethics other than technical, administrative, or other non-substantive amendments, or grant any waivers, including implicit waivers, from a provision of our Code of Ethics to our Chief Executive Officer, Chief Financial Officer, or certain other finance executives, we will disclose the nature of the amendment or waiver, its effective date and to whom it applies in a Current Report on Form 8-K filed with the SEC.
Board and Committee Meetings
Our board of directors held six (6) formal meetings and several informal meetings during the year ended August 31, 2021. All proceedings of the board of directors taken at a formal meeting were evidenced by way of minutes taken at such meetings. All other matters approved by the board of directors outside of any formal meeting were evidenced by resolutions consented to by all the directors. Such resolutions consented to in writing by the directors entitled to vote on that resolution at a meeting of the directors are, according to the Nevada General Corporate Law and our Bylaws, as valid and effective as if they had been passed at a meeting of the directors duly called and held.
Nomination Process
As of August 31, 2021, the Company had an active Governance and Nominating Committee. If shareholders wish to recommend candidates for our board of directors, they may do so by sending communications to the Governance and Nominating Committee at the address on the cover of this annual report.
Audit and Finance Committee and Audit Committee Financial Expert
The audit and finance committee is governed by the audit and finance committee charter, the most recent version having been adopted on December 8, 2020. Our audit and finance committee is currently composed of Mr. Al Reese, Jr., Mr. Ted McKechnie, and Mr. Nicholas Baxter. Mr. Reese, a CPA, qualifies as an "audit committee financial expert" as defined in Item 407(d)(5)(ii) of Regulation S-K, and is "independent" as the term is used in Item 7(d)(3)(iv) of Schedule 14A under the Securities Exchange Act of 1934, as amended. Prior to Mr. Reese’s appointment in January 2021, Mr. Bunka acted as a member of the audit and finance committee and was not “independent” pursuant to Nasdaq independence standards as he is actively involved in the daily management of the Company as CEO.
It is not the duty of our audit and finance committee to determine that our financial statements are complete and accurate and in accordance with generally accepted accounting principles. Our management is responsible for preparing our financial statements, and our independent registered public accounting firm is responsible for auditing those financial statements. Our audit and finance committee does, however, consult with management and our independent registered public accounting firm prior to the presentation of financial statements to shareholders and, as appropriate, initiates inquiries into various aspects of our financial affairs. In addition, our audit and finance committee is responsible for retaining, evaluating and, if appropriate, recommending the termination of our independent registered public accounting firm and approving professional services provided by them.

| Page 80 of 90 |
| Table of Contents |
Compensation Committee
The Company created a compensation committee on July 2, 2020, the members of which are Mr. Baxter, and Mr. McKechnie, with both Directors being “independent” pursuant to Nasdaq independence standards. The compensation committee operates under a written charter and its purpose is to review, consider, research, and recommend compensation for the Company’s executive management, taking into consideration achieved milestones, the compensation issued by companies of similar size and the overall financial health of the Company. The committee is also responsible for approving and reviewing employment agreements and benefits agreements as well as any executive compensation information incorporated into the Company’s periodic reports.
Governance and Nominating Committee
The governance and nominating committee operate pursuant to a written charter created on December 8, 2020, and subsequently adopted by the Board of directors. The current members of the committee are Mr. Reese Jr. and Mr. Baxter, both of whom are independent directors of the Company. The purpose of the committee is to assist the Board of directors with fulfilling its responsibilities by: (i) being satisfied that corporate governance guidelines are adopted, disclosed and applied including director qualification standards, director responsibilities, director access to management and independent advisors, director compensation, director orientation and continuing education, and annual performance evaluation of the Board; (ii) identifying individuals qualified to become new Board members and recommending to the Board the nominees for each annual meeting of shareholders of the Corporation; and (iii) such other matters delegated to the committee by the Board. A copy of the Governance & Nominating Committee charter can be downloaded from the Company’s website under our Investors/Governance/Governance Documents tab.
The Board of directors has a critical role in guiding our strategic direction and overseeing the management of our business, and accordingly, we seek to attract and retain highly qualified directors who have sufficient time to engage in the activities of the Board of directors and to understand and enhance their knowledge of our industry and business plans. In evaluating the suitability of individual candidates, the governance and nominating committee and Board of directors may take into account many factors, including: relevant education, experience and expertise; knowledge of the Company and the issues facing the Company; whether the candidate will strengthen the Board, as a whole, and remedy any perceived deficiencies in the specific criteria; moral and ethical character; diversity of expertise and experience in substantive matters pertaining to our business relative to other board members; diversity of background and perspective, including, but not limited to, with respect to age, gender, race, place of residence and specialized experience; and any other relevant qualifications, attributes or skills. The core competencies of directors should address accounting or finance experience, market familiarity, business or management experience, industry knowledge, customer-base experience or perspective, crisis response, leadership, and/or strategic planning. The Board of directors and governance and nominating committee evaluate each individual in the context of the Board as a whole, with the objective of assembling a group that can best perpetuate the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas.
Item 11. Executive Compensation
The particulars of the compensation paid to the following persons:
a) | our principal executive officer; |
|
|
b) | each of our two most highly compensated executive officers who were serving as executive officers at the end of the years ended August 31, 2021, and August 31, 2020, and |
|
|
c) | up to two additional individuals for whom disclosure would have been provided under (b) but for the fact that the individual was not serving as our executive officer at the end of the years ended August 31, 2021, and August 31, 2020, |

| Page 81 of 90 |
| Table of Contents |
who we will collectively refer to as the named executive officers of our Company, are set out in the following summary compensation table, except that no disclosure is provided for any named executive officer, other than our principal executive officers, whose total compensation did not exceed $100,000 for the respective fiscal year:
SUMMARY COMPENSATION TABLE | |||||||||
Name and Principal Position | Year | Salary $ | Bonus | Stock Awards | Option Awards(5) $ | Non-Equity Incentive Plan Compensation | Non-Qualified Deferred Compensation Earnings $ | All Other Compensation | Total |
Christopher Bunka (1) | 2021 2020
| - -
| - -
| - -
| 119,630 153,065
| - -
| - -
| 374,486 300,802 | 494,116 453,867
|
John Docherty (2) President & Director | 2021 2020 | - - | - - | - - | 83,419 275,614 | - - | - - | 326,855 242,521 | 410,274 518,135 |
Greg Downey (3) Chief Financial Officer | 2021 2020 | 84,688 - | - - | - - | 34,844 - | - - | - - | - - | 119,352 - |
Allan Spissinger (4) former Chief Financial Officer | 2021 2020 | - - | - - | - - | - 143,886 | - - | - - | 109,579 121,664 | 109,579 265,550 |
(1) | Mr. Bunka was appointed as Chairman, President, Chief Executive Officer, and director on October 26, 2006. We pay Mr. Bunka a consulting fee through CAB Financial Services Ltd., where he is also the Chief Executive Officer. |
(2) | Mr. Docherty became President on April 15, 2015, and a director on April 29, 2016. We pay Mr. Docherty a consulting fee through his wholly owned company Docherty Management Ltd. |
(3) | Mr. Downey became Chief Financial Officer on April 15, 2021, and is considered an employee of the Company. |
(4) | Mr. Spissinger became Chief Financial Officer on June 1, 2018. Mr. Spissinger was replaced as CFO effective April 15, 2021, and remained with the company until the end of his contract on May 31, 2021. We paid Mr. Spissinger a consulting fee through his wholly owned company M&E Services Ltd. |
(5) | The fair value of the stock options awarded was estimated using the Black-Scholes option pricing model. |
Consulting and Employment Agreements
Mr. Chris Bunka, CEO
The Company negotiated a 3-year term renewable management contract with Mr. Bunka effective January 1, 2019. The base annual compensation payable is C$350,000 per year with an annual increase of 1.25 times the annual Canadian inflation rate. A performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the board of directors and he is also entitled to participate in the Company’s approved stock option plan.
Mr. Bunka is entitled to compensation equal to 2% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. Upon the occurrence of a change of control, subject to certain exemptions, Mr. Bunka will also be entitled to a lump payment of twenty-three times his monthly fee. The termination clause of Mr. Bunka contract states that three (3) months notice must be given for terminating his contract without cause. Given such notice, the Company would be liable for a termination break fee payment equal to fifteen (15) times his monthly fee.

| Page 82 of 90 |
| Table of Contents |
As at the date of this report, the compensation committee is in negotiations with Mr. Bunka for the renewal of his existing contracts.
Mr. John Docherty, President
The Company has an agreement with Docherty Management Limited, solely owned by Mr. John Docherty for a 3-year term renewable management contract for C$300,000 per year, effective January 1, 2019, with an annual increase of 1.25 times the annual Canadian inflation rate. A performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the board of directors and he is also entitled to participate in the Company’s approved stock option plan. An annual professional development allowance of C$15,000 is also available to Mr. Docherty.
The contracts for the services of the President also include the following performance incentives: entitlement to compensation equal to 2% of the consideration received by the Company from the sale of a subsidiary, excluding certain circumstances. Upon the occurrence of a change of control, subject to certain exemptions, Mr. Docherty will also be entitled to a lump payment of twelve (12) times his monthly fee. The contract specifies that termination without cause would result in eight (8) months pay in leu of notice.
As at the date of this report, the compensation committee is in negotiations with Mr. Docherty for the renewal of his existing contract.
Mr. Greg Downey, CFO
On April 15, 2021, the Company entered into an employment contract with Mr. Downey as Chief Financial Officer with annual compensation of C$144,000 with a 10% annual increase. A performance bonus equal to 50% of the annual compensation may be payable upon the completion of certain performance criteria as determined by the board of directors and he is also entitled to participate in the Company’s approved stock option plan. An annual professional development allowance of C$5,000 is also available to Mr. Downey.
Mr. Downey is eligible for incentive compensation of 1% of the consideration received by the Company from the sale of a subsidiary excluding certain circumstances. Upon the occurrence of a change of control, Mr. Downey will also be entitled to a lump payment of sixteen (16) times his monthly salary.
The contract specifies that termination without cause clause would result in eight (8) months pay in leu of notice.
Other than as set out in this annual report on Form 10-K we have not entered into any employment or consulting agreements with any of our current officers or directors.
Grants of Plan-Based Awards Table
During the fiscal year ended August 31, 2021, Lexaria issued the following plan-based awards to our named executive officers:

| Page 83 of 90 |
| Table of Contents |
Compensation Securities | |||||||
Executive Officer | Type of compensation security | Number of compensation securities, number of underlying securities, and percentage of class | Date of issue or grant | Issue, conversion or exercise price $ | Closing price of security or underlying security on date of grant $ | Closing price of security or underlying security at year end $ | Expiry date |
Chris Bunka, CEO | Stock Options | 26,000 | 04/25/2021 | 5.83 | 5.33 | 6.22 | 04/25/2026 |
John Docherty, President | Stock Options | 18,000
| 04/25/2021 | 5.31
| 5.33 | 6.22 | 04/25/2026
|
Greg Downey, CFO | Stock Options | 12,000 10,000 | 04/15/2021 04/25/2021 | 5.04 5.31 | 5.07 5.33 | 6.22 | 04/15/2026 04/25/2026 |
Outstanding Equity Awards at Fiscal Year End
The particulars of unexercised options, stock that has not vested and equity incentive plan awards for our named executive officers are set out in the following table:
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | ||||||||||
| OPTION AWARDS | STOCK AWARDS | ||||||||
Executive Officer | Number of Securities Underlying Exercisable (#) | Number of Securities Underlying Unexercised Options Unexercisable (#) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested $ | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) | |
Christopher Bunka | 26,000 23,334 | - - | - - | $5.83 $7.08 | 04/23/2026 06/08/2026 | - - | - - | - - | - - | |
John Docherty | 13,334 18,000 18,334 | - - - | - - - | $9.60 $5.31 $7.08 | 04/23/2025 04/23/2026 06/08/2026 | - - - | - - - | - - - | - - - | |
Greg Downey | 12,000 5,000 8,000 | 8,000 5,000 - | - - - | $5.04 $5.31 $7.08 | 04/24/2026 04/25/2026 06/08/2026 | - - - | - - - | - - - | - - - | |

| Page 84 of 90 |
| Table of Contents |
Option Exercises
No options were exercised by any named executive officer during our fiscal year ended August 31, 2021.
Compensation of Directors
As of August 31, 2021, three of our directors are compensated for their services. In their capacity as independent directors each receives $30,000 per year paid quarterly in advance. Additionally, directors are paid nominal amounts for their services on the audit and finance, compensation, and the governance and nominating committees and for acting as chair of such committees.
During the year ended August 31, 2021, three of our directors were granted an aggregate of 6,400 stock options with a fair value calculated at $75,540 and included in consulting expense.
Pension, Retirement or Similar Benefit Plans
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We have no material bonus or profit-sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that stock options may be granted at the discretion of the board of directors or a committee thereof.
Indebtedness of Directors, Senior Officers, Executive Officers and Other Management
None of our directors or executive officers or any associate or affiliate of our company during the last two fiscal years is or has been indebted to our Company by way of guarantee, support agreement, letter of credit or other similar agreement or understanding currently outstanding.
Compensation Committee Interlocks and Insider Participation
No member of the Compensation Committee is, or was during fiscal 2021, an officer or employee of the Company or any of its subsidiaries or was formerly an officer of the Company or any of its subsidiaries. No member of the Compensation Committee is, or was during fiscal 2021, an executive officer of another company whose board of directors has a comparable committee on which one of the Company’s executive officers serves.
Board Diversity
The Company and its management are highly supportive of the recent initiatives taken by the Securities and Exchange Commission and the Nasdaq Group to encourage diversity within the board of directors of reporting companies. Lexaria annually reviews its board composition and evaluates areas of expertise that would provide additional benefits to the Company and its shareholders. As the Company transitions its technology towards pharmaceutical applications, should the Company feel it is beneficial to expand its board, the Company will endeavour to engage individuals who will be able to enhance the board with their expertise in this industry sector and who also will enrich the board with their diverse perspectives.
Compensation Committee Report
Our Compensation Committee has reviewed and discussed the Executive Compensation for the year ended August 31, 2021, with management. Based on the reviews and discussions our Compensation Committee recommended to our Board of directors that the Executive Compensation discussed above to be included in our filing of our annual report on Form 10-K for the year ended August 31, 2021.

| Page 85 of 90 |
| Table of Contents |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth, as of August 31, 2021, certain information with respect to the beneficial ownership of our common shares by each shareholder known by us to be the beneficial owner of more than 5% of our common shares, as well as by each of our current directors and executive officers as a group. Each person has sole voting and investment power with respect to the shares of common stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of common stock, except as otherwise indicated.
|
| Amount and Nature of Beneficial Ownership |
|
| Percentage |
| ||
Christopher Bunka; Kelowna BC, Canada |
|
| 545,455 | (1) |
|
| 9.52 | % |
John Docherty; Toronto, ON, Canada |
|
| 103,743 | (2) |
|
| 1.81 | % |
Greg Downey; Kelowna, BC, Canada * |
|
| 26,883 | (3) |
|
| 0.47 | % |
Ted McKechnie; Toronto, ON, Canada * |
|
| 19,691 | (4) |
|
| 0.34 | % |
Nicholas Baxter; Aberdeenshire, UK * |
|
| 17,500 | (5) |
|
| 0.31 | % |
Al Reese Jr., Houston, TX, USA * |
|
| 4,137 | (6) |
|
| 0.08 | % |
Directors and Executive Officers as a Group (6 persons) |
|
| 717,539 |
|
|
| 12.53 | % |
* | Less than 1% beneficial ownership |
(1) | Chairman, director and CEO Chris Bunka directly held 273,543 shares and a further 215,912 shares held in C.A.B. Financial Services. Also included in his holdings are 23,333 warrants exercisable at $10.50 and 26,000 options exercisable at $5.83 and 23,334 at $7.08. |
(2) | President and Director John Docherty holdings include 13,334 options exercisable at $9.60, 18,000 at $5.31, and 18,334 at $7.08. |
(3) | CFO Greg Downey holdings include 12,000 options exercisable at $5.04, 5,000 at $5.31, and 8,000 at $7.08. |
(4) | Director Ted McKechnie holdings includes 1,500 options exercisable at $5.31 and 5,000 at $7.08. |
(5) | Director Nicholas Baxter holdings includes 1,500 options exercisable at $5.31 and 5,000 at $7.08. |
(6) | Director Al Reese Jr. holdings include Includes 3,400 options exercisable at $4.80. |
Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person’s actual ownership or voting power with respect to the number of shares of common stock actually outstanding on November 26, 2021. As of November 26, 2021, there were 5,726,699 shares of our common stock issued and outstanding.
Changes in Control
We are unaware of any contract or other arrangement the operation of which may at a subsequent date result in a change in control of our Company.

| Page 86 of 90 |
| Table of Contents |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Except as disclosed herein, no director, executive officer, shareholder holding at least 5% of shares of our common stock, or any family member thereof, had any material interest, direct or indirect, in any transaction, or proposed transaction since the year ended August 31, 2021, in which the amount involved in the transaction exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at the yearend for the last three completed fiscal years.
Director Independence
We currently act with five directors, consisting of Mr. Christopher Bunka, Mr. John Docherty, Mr. Nicholas Baxter, Mr. Ted McKechnie, and Mr. Al Reese Jr. We have determined that Mr. Baxter, Mr. McKechnie, and Mr. Reese are “independent directors” as defined in Nasdaq Marketplace Rule 4200(a)(15).
Currently our audit and finance committee consists of our Mr. Baxter, Mr. McKechnie, and Mr. Reese, who qualifies as an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K.
From inception to present date, we believe that the members of our audit committee and the board of directors have been and are collectively capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting.
We appointed a compensation committee on July 2, 2020, which currently consists of the following independent directors: Mr. McKechnie, and Mr. Baxter. During fiscal year ended August 31, 2021, the compensation committee held one meeting to determine bonus compensation payable to the named executive officers in connection with the successful completion of certain performance milestones and the disposition of assets of CanPharm.
We appointed a governance and nominating committee on December 8, 2020 which currently consists of the following independent directors: Mr. Reese Jr. and Mr. Baxter. To date no meetings have been held by this committee.
Item 14. Principal Accounting Fees and Services
The aggregate fees billed for the most recently completed fiscal year ended August 31, 2021, and for fiscal year ended August 31, 2020 for professional services rendered by the principal accountant for the audit of our annual financial statements and review of the financial statements included in our quarterly reports on Form 10-Q and services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for these fiscal periods were as follows:
|
| Year Ended |
| |||||
|
| August 31, 2021 |
|
| August 31, 2020 |
| ||
Principal Accounting Fees |
| $ |
|
| $ |
| ||
Audit |
|
| 73,733 |
|
|
| 67,450 |
|
Audit Related |
|
| 18,458 |
|
|
| - |
|
Tax |
|
| - |
|
|
| - |
|
Total |
|
| 92,191 |
|
|
| 67,450 |
|
Audit Fees: Audit fees consist of fees billed for professional services rendered for the audits of our financial statements, reviews of our interim financial statements included in quarterly reports, services performed in connection with filings with the Securities and Exchange Commission and related comfort letters and other services that are provided by the Company’s principal accountants for the fiscal years ended August 31, 2021 and August 31, 2020 in connection with statutory and regulatory filings or engagements.

| Page 87 of 90 |
| Table of Contents |
Audit related Fees: Audit related fees consist of fees billed for assurance and related services by the Company’s principal accountant that are reasonably related to the performance of the audit or review of the Company’s financial statements, which are not included in the Audit Fees described above.
Tax Fees: Tax fees consist of fees billed for professional services for tax compliance, tax advice and tax planning. These services include assistance regarding federal, state and local tax compliance and consultation in connection with various transactions and acquisitions.
We do not use our principal accountants for services other than those relative to our annual audit and the review of our interim financial statements and certain SEC filings. We therefore do not involve our principal accountants for matters related to tax compliance and financial information system design and implementation. These services, including corporate tax preparation and the designing or implementing of a system that aggregates source data underlying the financial statements or generates information that is significant to our financial statements, are provided internally or by other service providers.
Effective May 6, 2003, the SEC adopted rules that require that before our independent auditors are engaged by us to render any auditing or permitted non-audit related service, the engagement be:
· | approved by our audit committee; or |
|
|
· | entered into pursuant to pre-approval policies and procedures established by the board of directors, provided the policies and procedures are detailed as to the particular service, the board of directors is informed of each service, and such policies and procedures do not include delegation of the board of directors' responsibilities to management. |
Our board of directors pre-approves all services provided by our independent auditors. All of the above services and fees were reviewed and approved by the board of directors either before or after the respective services were rendered.
Our board of directors has considered the nature and amount of fees billed by our independent auditors and believes that the provision of services for activities unrelated to the audit is compatible with maintaining our independent auditors’ independence.
PART IV
Item 15. Exhibits, Financial Statement Schedules
a) Financial Statements
1) | Financial statements for our Company are listed in the index under Item 8 of this document. |
|
|
2) | All financial statement schedules are omitted because they are not applicable, not material or the required information is shown in the financial statements or notes thereto. |

| Page 88 of 90 |
| Table of Contents |
b) Exhibits
Exhibit Number |
| Description |
(2) |
| Plan of Acquisition, Reorganization, Arrangement, Liquidation or Succession |
| Plan of Conversion (included as Schedule “A” to the proxy statement/prospectus) | |
(3) |
| Articles of Incorporation and Bylaws |
| ||
| ||
| Amended and Restated Articles of Incorporation (Filed on Form 8-K January 14, 2021 Exh. 3.1) | |
| ||
| Amended and Restated Bylaws (Filed on Form S-1 June 3, 2020 Exh 3.4) | |
| ||
| Amendment to Articles of Incorporation – Share Expansion (Filed on Form 8-K March 10th, 2010) | |
| ||
| Amendment to Articles of Incorporation – Name Change (Filed on Form 8-K May 11th, 2016 Exh 99.1) | |
(4) |
| Instruments Defining the Rights of Security Holders, including Indentures |
| ||
(10) |
| Material Contracts |
| ||
| ||
(21) |
| Subsidiaries |
| ||
(23) |
| Consents of Experts and Counsel |
| Consent of Davidson & Company LLP, Chartered Professional Accountants | |
(31) |
| Rule 13(a) - 14 (a)/15(d) - 14(a) |
| Section 302 Certifications under Sarbanes-Oxley Act of 2002 of Principal Executive Officer | |
| ||
(32) |
| Section 1350 Certifications |
| Section 906 Certification under Sarbanes Oxley Act of 2002 of Principal Executive Officer | |
| ||
(101)** |
| Interactive Data Files |
101.INS |
| XBRL Instance Document |
101.SCH |
| XBRL Taxonomy Extension Schema Document |
101.CAL |
| XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
| XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
| XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
| XBRL Taxonomy Extension Presentation Linkbase Document |
*Incorporated by reference to same exhibit filed with the Company's Registration Statement on Form SB-2 filed March 1, 2006.
** Furnished herewith. Pursuant to Rule 406T of Regulation S-T, the Interactive Data Files on Exhibit 101 hereto are deemed not filed or part of any registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, are deemed not filed for purposes of Section 18 of the Securities and Exchange Act of 1934, and otherwise are not subject to liability under those sections.

| Page 89 of 90 |
| Table of Contents |
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
LEXARIA BIOSCIENCE CORP.
By: | /s/ Christopher Bunka |
|
Christopher Bunka
Chief Executive Officer, Chairman and Director
(Principal Executive Officer)
Date: November 26, 2021
In accordance with the Exchange Act, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
By: | /s/ Christopher Bunka |
|
Christopher Bunka
Chief Executive Officer, Chairman and Director
(Principal Executive Officer)
Date: November 26, 2021
By: | /s/ John Docherty |
|
John Docherty
President and Director
Date: November 26, 2021
By: | /s/ Gregory Downey |
|
Gregory Downey CPA, CMA
Chief Financial Officer
(Principal Financial Officer)
Date: November 26, 2021
By: | /s/Ted McKechnie |
|
Ted McKechnie
Director
Date: November 26, 2021
By: | /s/Nicholas Baxter |
|
Nicholas Baxter
Director
Date: November 26, 2021
By: | /s/Albert Reese Jr. |
|
Albert Reese Jr.
Director
Date: November 26, 2021

| Page 90 of 90 |